No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
Generic Vaniqa
New
Generic Vaniqa is available under the name Eflora cream made by Ranbaxy, world-known pharmaceutical producer. Women use generic Vaniqa to reduce excessive hair growth on the face. Vaniqa is a cream specially developed for women with excessive hair growth on the face (neck, chin, and upper lip area).
277 Items
More info


|
Basic Information
|
 |
Eflornithine (Vaniqa generic) - best facial hair removal cream!
Are you still troubles by unwanted facial hair growth?
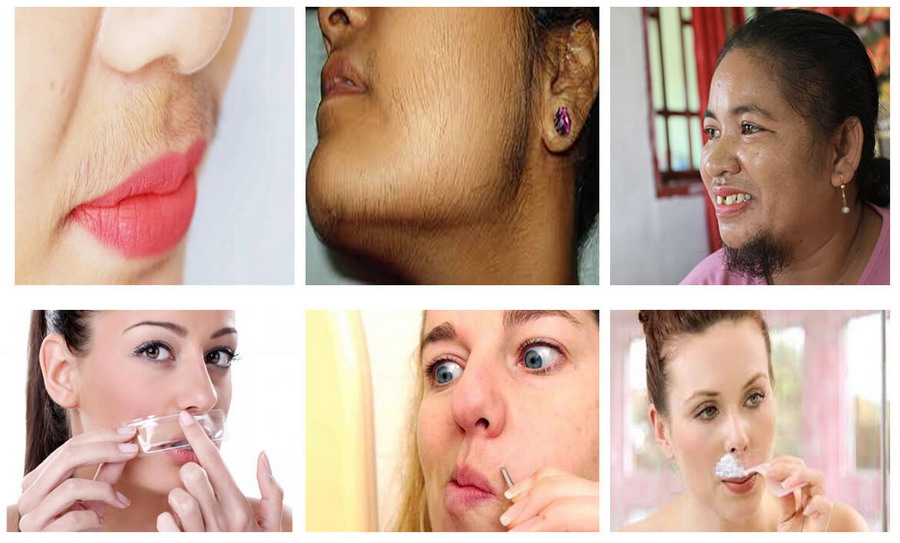
Do you still resolve the problem by shaving and plucking mustache?
Eflora (generic Vaniqa) your product of choice for unwanted facial hair
Eflornithine is a clinically proven cream that reduces facial hair growth by 70%. Eflornithine works by blocking the activity of the enzyme ornithine decarboxylase found in the hair follicles and essential for hair growth.
Eflora only slows down hair growth. It does not remove hair, so other hair removal methods such as plucking and shaving must be continued while using it. The results of using Vaniqa cream are usually visible within the first two months after starting treatment.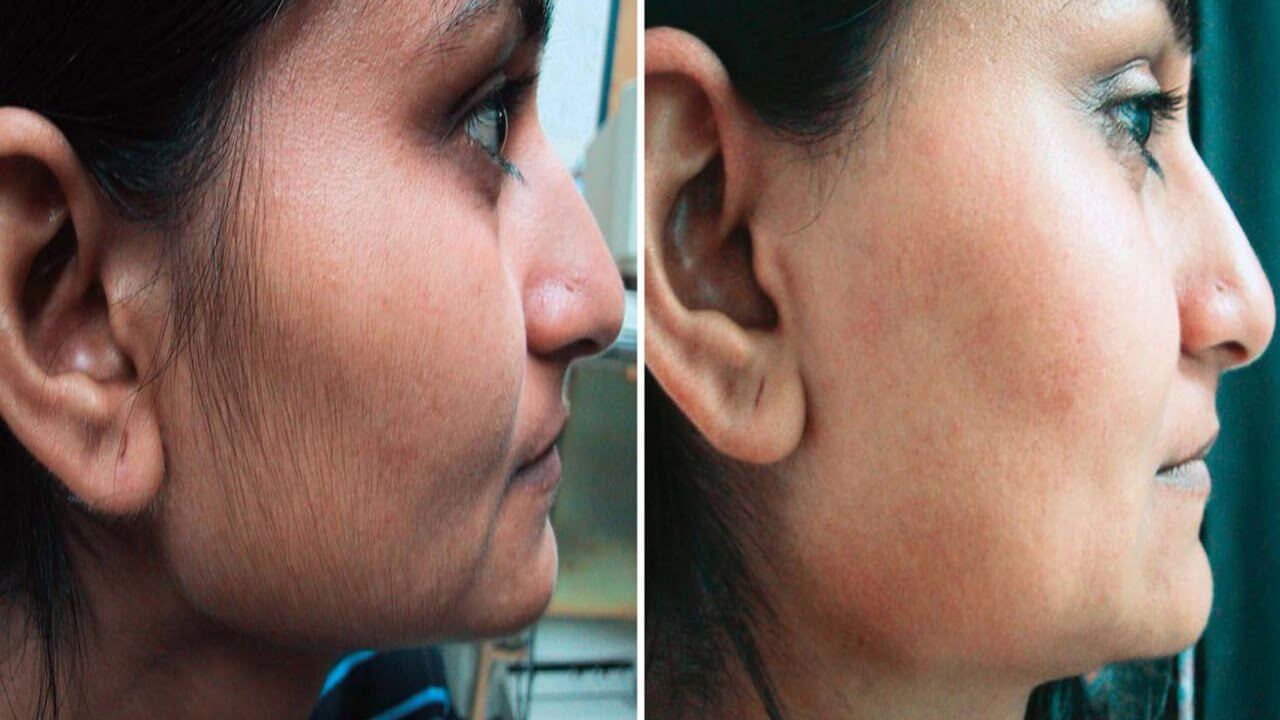

|
Why do you need to use generic Vaniqa? |
||||
|
 |
|||
1. Apply a thin layer of Eflora cream to the affected area of the face and rub it gently.
2. Apply cream twice daily with an interval of at least 8 hours.
3. Do not wash your face at least 4 hours after application.
4. Apply cream each day preferably at the same time each day. The first result may occur within 4 weeks.
5. A woman should continue using hair removal techniques in combination with Eflora cream.
6. Use Eflora before applying moisturizers, sunscreens, and cosmetics.
7. Use Eflora cream only on the face affected areas.

Monthly progress with Eflora
The results may vary depending on many factors such as age, condition, and many others. According to the results of clinical studies, first visible results are achieved within 4 weeks.
- 1-3 weeks - start your treatment. Usually, the results are not visible. Be patient with your progress.
- 3-4 weeks - you may see the first results. Hair growth is reduced. You may notice an improved appearance.
- 4-8 weeks - the desired effects are usually achieved during this period. Most women notice a significant reduction of facial hair growth.
- 8-24 weeks - the effect is maintained. Side effects are not usually observed.
Eflora (generic Vaniqa) helps significantly reduce the rate of unwanted facial hair growth. Excessive hair growth usually disappears within 2 months.
Full satisfaction is guaranteed!!!

Vaniqa and Eflora are identical products made by two different manufacturers. They contain the same active ingredient Eflornithine hydrochloride and are used for the same purposes. Both products provide great results in facial hair removal cream.
- Vaniqa=Eflora
- Same active ingredients
- Same additives
- Same volume
- Same indications
- Same results for less money

Generic Vaniqa brief description.
Product name - Generic Vaniqa - Eflora cream - Eflornithine cream.
Qualitative and quantitative composition - 1 gm of Vaniqa is composed of 115 mg hydrochloride monohydrate of eflornithine. The other ingredients are stearyl alcohol; purified water; Propyl 4-hydroxybenzoate; polyethylene glycol stearate; Phenoxyethanol; Paraffin oil; Methyl 4-hydroxybenzoate; macrogol-cetyl-stearyl ether; Glyceryl stearate; dimethicone; and cetostearyl alcohol.
Action mechanism - Generic Vaniqa slows hair growth by acting on an enzyme involved in hair formation. It inhibits the enzyme ornithine decarboxylase which catalyzes the conversion of ornithine into various polyamines that regulate cell growth and cell differentiation in the hair follicle. This slows down the formation of the hair.
It is not for hair removal. As it is not a hair removal cream, it may be necessary to continue removing hair, for example by shaving or plucking. An improvement can already be seen 2 months after the start of treatment. If you do not find relief after 4 months of treatment, please consult your doctor. If you stop the application, the original hair growth can recur within 2 months.
Indication - The cream is indicated to women with excess hair growth in the neck, chin and upper lip area. It controls hair growth only in these areas and not any other part of the body.
Mode of administration - Generic Vaniqa cream (Eflora) should be applied to the affected skin areas at least twice a day with a minimum gap of 8 hours between applications. The product is effective when used in the face or below the chin region. The application should be limited to these areas. The maximum use of the medicine should not exceed 30 gm per month. An improvement in the condition can be detected within eight weeks after the start of therapy.
Contraindication - Hypersensitivity to the active substance or to any of the other ingredients of the medicine.
Special cautions and warnings - Do not use Vaniqa if you are allergic to Eflornithine or any of the other ingredients of the medicine. If you have liver or kidney disorder, refrain from using it. Do not put the cream in the eyes, the inside of the nose or mouth. Excessive hair growth can be caused by underlying diseases such as androgens producing neoplasm, polycystic ovarian syndrome, or certain medicines including phenytoin, phenobarbital, minoxidil, glucocorticoids, cyclosporin, combined Estrogen-androgen hormone replacement therapy. Contact your doctor if you have any questions.
Vaniqa is intended for use on the skin only. Contact with eyes or Mucous membranes (eg nose or mouth) should be avoided. When applying the cream on sore or cracked skin, it may cause a temporary stinging or burning sensation.
If skin irritation persists for more than a day, treatment should be discontinued and you should consult your doctor.
Interaction - To date, no interaction with other drugs have been established
Side effects - Like all other medicines, Vaniqa can cause side effects. The most commonly reported generic Vaniqa side effect is acne (7 to 14%). Other common side effects include (> 1%) skin problems such as skin reaction from growing hair; hair loss; burning, stinging or tingling sensation; dry skin; itching, redness or rash.
In addition, occasional (<1%) side effects reported are ingrown of hair; swelling of the face or mouth; bleeding from the skin; cold sores; dry, chapped or numb lips, paleness, soreness, and redness of the skin; eczema; skin irritation; hair shaft anomaly; or abnormal hair growth.
Rare (<0.1%) side effects seen in some individuals are rosacea (inflammation and redness of the skin on the face, sometimes purulent); a red, scaly, itchy dermatitis; skin growths; red raised rash; cysts; blistered rash; excessive hair growth; tightening of the skin, and other skin disorders.
If you notice any of these or any other side effects, or if you are unsure about the effects of Vaniqa, contact your doctor or pharmacist.
Interesting facts
-
Vaniqa generic is a white to off-white cream
-
Do not store the tube above 25°C
-
Wait at least 8 hours between 2 successive applications
-
Do not swallow the medicine
-
It can take up to 2 months to see an effect. Continued use of the drug without interruption may result in further improvement. In order to maintain the success of the treatment, you must use this medicine permanently.
-
After stopping the treatment, the condition can return back to the original state after 8 weeks
-
Eflornithine acts directly on the hair root, stopping the hair growth. In contrast to the usual depilatory creams from the drugstore, no aggressive chemicals are used. There is no chemical action on the skin surface, thus causing less skin irritation. The cream must be applied regularly for 6 to 8 weeks for a noticeable change to take place.
Generic Vaniqa Real Shots
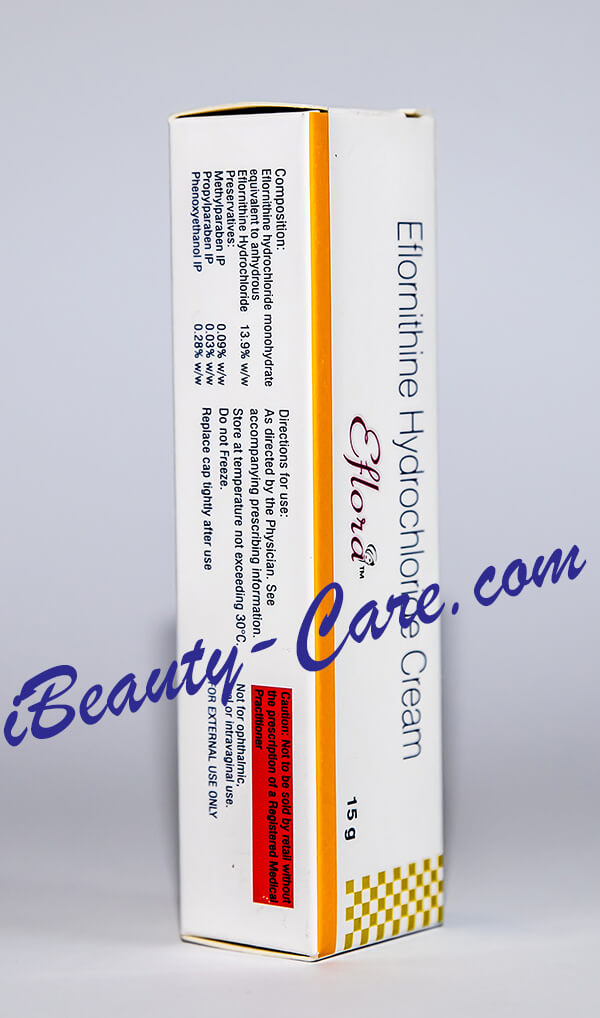


Full Original Product Annotation
 GENERIC NAME
GENERIC NAME
Generic Vaniqa / Eflornithine Hydrochloride Cream
COMPOSITION:
Eflornithine hydrochloride monohydrate Equivalent to anhydrous
Eflornithine Hydrochloride13.9% w/w
Preservatives:
Methylparaben IP 0.09%w/w
Propylparaben IP 0.03%w/w
Phenoxyethanol IP0.28%w/w
DOSAGE FORM
Cream
DESCRIPTION
Generic Vaniqa (Eflornithine hydrochloride) is a cream containing 13.9% (139 mg/g) of anhydrous Eflornithine hydrochloride as Eflornithine hydrochloride monohydrate (150mg/g). Chemically, Eflornithine hydrochloride is (±) -2- (difluoromethyl) ornithine monohydrochloride monohydrate, with the empirical formula C6H12F2N2O2.HCL.H2O, a molecular weight of 236.65 and the following structural formula: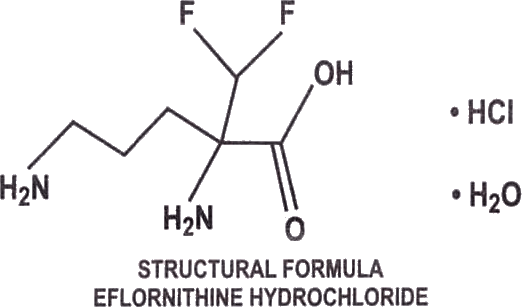
INDICATIONS
Generic Vaniqa cream is indicated for the reduction of unwanted facial hair in women.
Eflornithine cream has only been studied on the face and adjacent involved areas under the chin of affected individuals. Usage should be limited to these areas of involvement.
DOSE AND METHOD OF ADMINISTRATION
Apply a thin layer of EFLORA (Eflornithine hydrochloride) cream, 13.9% to affected areas of the face and adjacent involved areas under the chin and rub in thoroughly. Do not wash treated area for at least 4 hours. Apply the cream twice daily at least 8 hours apart or as directed by a physician. The patient should continue to use hair removal techniques as needed in conjunction with EFLORA cream (EFLORA cream should applied at least 5 minutes after hair removal). Cosmetics or sunscreens may be applied over treated areas after cream has dried.
USE IN SPECIAL POPULATIONS
Pregnancy
Teratogenic effects Pregnancy Category C
In the first dermal embryo-fetal development study in rats treated with Eflornithine hydrochloride cream, 13.9% (in which no precautions were taken to prevent ingestion of drug from application sites), maternal toxicity and fetal effects including reduced numbers of live fetuses, decreased fetal weights, and delayed ossification and development of the viscera were observed at doses of 225 and 450 mg/kg (15X and 29 X the MRHD based on BSA, respectively). When the study was repeated under conditions that avoided ingestion from application sites, no maternal, fetal or teratogenic effects were observed at doses up to 450 mg/kg (29 X the MRHD based on BSA). In the first study in which no precautions were taken to prevent ingestion, circulating plasma levels were 11- to 14-fold higher than in the second study in which ingestion was prevented. In a dermal embryo-fetal development study in rabbits treated with Eflornithine no adverse maternal or fetal effects occurred at doses up to 90 mg/kg (11 X the MRHD based on BSA). Significant dermal irritation, as well as possible ingestion of Eflornithine occurred at 300 mg/kg/day (36 X the MRHD based on BSA) and was associated with maternal deaths, abortions, increased fetal resorptions, and reduced fetal weights. Fetotoxicity in the absence of maternal toxicity has been reported in oral studies with Eflornithine with fetal no-effect doses of 80 mg/kg in rats and 45 mg/kg in rabbits. In these studies, no evidence of teratogenicity was observed in rats given up to 200 mg/kg or in rabbits given up to 135 mg/kg.
Although Eflornithine was not formally studied in pregnant patients, 22 pregnancies occurred during the trials. Nineteen of these pregnancies occurred while patients were using Eflornithine. Of the 19 pregnancies, there were 9 healthy infants, 4 spontaneous abortions, 5 induced/elective abortions, and 1 birth defect (Down's Syndrome to a 35- year-old).
Because there are no adequate and well-controlled studies in pregnant women, the risk/benefit ratio of using Eflornithine in women with unwanted facial hair who are pregnant should be weighed carefully with serious consideration for either not implementing or discontinuing use of Eflornithine.
Lactation
It is not known whether or not Eflornithine hydrochloride is excreted in human milk. Caution should be exercised when Eflornithine is administered to a nursing woman.
Pediatrics
The safety and effectiveness of Eflornithine have not been established in pediatric patients less than 12 years of age.
Geriatrics
Of the 1373 patients on active treatment in clinical studies of Eflornithine, approximately 7% were 65 years or older and approximately 1% were 75 or older. No apparent differences in safety were observed between older patients and younger patients.
CONTRAINDICATIONS
EFLORA cream is contraindicated patients with a known hypersensitivity to Eflornithine or any inactive ingredients of the cream.
WARNINGS
Discontinue use if hypersensitivity occurs.
Excessive hair growth can result from serious underlying disorders (e.g. polycystic ovary syndrome, androgen secreting neoplasm) or certain medications (e.g. cyclosporin, glucocorticoids, minoxidil, phenobarbitone, phenytoin, combined oestrogen-androgen hormone replacement therapy). These factors should be considered in the overall medical treatment of patients who might be prescribed Eflornithine.
As the safety of Eflornithine has not been studied in patients with severe renal impairment, caution should be used when prescribing Eflornithine for these patients.
PRECAUTIONS
Eflornithine is for cutaneous use only. Contact with eyes or mucous membranes (e.g. nose or mouth) should be avoided. Transient stinging or burning may occur when the cream is applied to abraded or broken skin.
If skin irritation or intolerance develops, the frequency of application should be reduced temporarily to once a day. If irritation continues, treatment should be discontinued and the physician consulted.
It is recommended that hands are washed following use.
Information for Patients
Patients using Eflornithine should receive the following information and instructions:
This medication is not a depilatory, but rather appears to retard hair growth to improve the condition and the patient's appearance. Patients will likely need to continue using a hair removal method (e.g., shaving, plucking, etc.) in conjunction with Eflornithine hydrochloride Cream, 13.9%.
Onset of improvement was seen after as little as 4-8 weeks of treatment in the 24-week clinical trials. The condition may return to pretreatment levels 8 weeks after discontinuing treatment.
If skin irritation or intolerance develops, direct the patient to temporarily reduce the frequency of application (e.g., once a day). If irritation continues, the patient should discontinue use of the product.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 12-month photocarcinogenicity study in hairless albino mice, animals treated with the vehicle alone showed an increased incidence of skin tumors induced by exposure to ultraviolet (UVA/UVB) light, whereas mice treated topically with Eflornithine at doses up to 600 mg/kg [19 X the Maximum Recommended Human Dose (MRHD) based on body surface area (BSA)] showed an incidence of skin tumors equivalent to untreated-control animals. A two- year dermal carcinogenicity study in CD-1 mice treated with Eflornithine revealed no evidence of carcinogenicity at daily doses up to 600 mg/kg (950 X the MRHD based on AUC comparisons).
Eflornithine did not elicit mutagenic effects in an Ames reverse-mutation assay or clastogenicity in primary human lymphocytes, with and without metabolic activation. In a dermal micronucleus assay, Eflornithine hydrochloride cream, 13.9%, at doses up to 900 mg/kg (58 X the MRHD based on BSA) in rats yielded no evidence of genotoxicity.
In a dermal fertility and early embryonic development study in rats treated with Eflornithine there were no adverse reproductive effects at doses up to 450 mg/kg (29 X the MRHD based on BSA). In a peri-and postnatal study in rats, Eflornithine administered in the drinking water was associated with maternal toxicity and reduced pup weights at doses of at least 625 mg/kg (40 X the MRHD based on BSA) and a slightly reduced fertility index, which was considered to be of questionable biological significance, at 1698 mg/kg (11 OX the MRHD based on BSA). No effects were seen with an oral dose of 223 mg/kg (14 X the MRHD based on BSA). In the latter study, the multiples of the human exposure are likely much higher, since Eflornithine is well absorbed orally in rats, whereas minimal absorption occurs in humans treated topically.
DRUG INTERACTIONS
It is not known if Eflornithine has any interaction with other topically applied drug products.
UNDESIRABLE EFFECTS
Adverse events reported for most body systems occurred at similar frequencies in Eflornithine and vehicle control groups. The most frequent adverse events related to treatment with Eflornithine were skin-related. The following table notes the percentage of adverse events associated with the use of Eflornithine or its vehicle that occurred at greater than 1% in both the vehicle-controlled studies and the open-label safety studies up to 1 year of continuous use.
|
Adverse Event Term |
Vehicle-Controlled Studies |
Vehicle- Controlled and Open-Label Studies |
|
|
Eflornithine 13.9%w/w (n=393) |
Vehicle (n=201) |
Eflornithine cream 13.9%w/w (n=1373) |
|
|
Acne |
21.3 |
21.4 |
10.8 |
|
Pseudofolliculitis Barbae |
16.3 |
15.4 |
4.9 |
|
Stinging Skin |
7.9 |
2.5 |
4.1 |
|
Headache |
3.8 |
5.0 |
4.0 |
|
Burning Skin |
4.3 |
2.0 |
3.5 |
|
Dry Skin |
1.8 |
3.0 |
3.3 |
|
Pruritus (itching) |
3.8 |
4.0 |
3.1 |
|
Erythema (redness) |
1.3 |
0.0 |
2.5 |
|
Tingling Skin |
3.6 |
1.5 |
2.2 |
|
Dyspepsia |
2.5 |
2.0 |
1.9 |
|
Skin Irritation |
1.0 |
1.0 |
1.8 |
|
Rash |
2.8 |
0.0 |
1.5 |
|
Alopecia |
1.5 |
2.5 |
1.3 |
|
Dizziness |
1.5 |
1.5 |
1.3 |
|
Folliculitis |
0.5 |
0.0 |
1.0 |
|
Hair Ingrown |
0.3 |
2.0 |
0.9 |
|
Facial edema |
0.3 |
3.0 |
0.7 |
|
Anorexia |
1.0 |
2.0 |
0.7 |
|
Nausea |
0.5 |
1.0 |
0.7 |
|
Asthenia |
0.0 |
1.0 |
0.3 |
|
Vertigo |
0.3 |
1.0 |
0.1 |
Treatment related skin adverse events that occurred in less than 1% of the subjects treated with eflornithine are: bleeding skin, cheilitis, contact dermatitis, swelling of lips, herpes simplex, numbness and rosacea.
Adverse events were primarily mild in intensity and generally resolved without medical treatment or discontinuation of Eflornithine. Only 2% of subjects discontinued studies due to an adverse event related to use of Eflornithine.
Laboratory Test Abnormalities
No laboratory test abnormalities have been consistently found to be associated with Eflornithine. In an open labeled study, some patients showed an increase in their transaminases; however, the clinical significance of these findings is not known
OVERDOSAGE
Overdosage information with Generic Vaniqa is unavailable. Given the low percutaneous penetration of this drug, overdosage via the topical route is not
expected. However, should very high topical doses (e.g., multiple tubes per day) or oral ingestion be encountered (a 30 g tube contains 4.2 g of Eflornithine hydrochloride), the patient should be monitored, and appropriate supportive measures administered as necessary.
(Note: Use of an intravenous formulation of Eflornithine hydrochloride at high doses (400 mg/kg/day or approximately 24 g/day) for the treatment of ' Trypanosoma brucei gambiense infection (African sleeping sickness) has been associated with adverse events and laboratory abnormalities. Adverse events in this setting have included hair loss, facial swelling, seizures, hearing = impairment, stomach upset, loss of appetite, headache, weakness and ' dizziness. A variety of hematological toxicities, including anemia, I thrombocytopenia and leukopenia have also been observed, but these were usually reversible upon discontinuation of treatment.)
PHARMACODYNAMIC AND PHARMACOKINETIC PROPERTIES
Mechanism of Action
There are no studies examining the inhibition of the enzyme ornithine decarboxylase (ODC) in human skin following the application of topical Generic Vaniqa. However, there are studies in the literature that report the inhibition of ODC activity in skin following oral Eflornithine. It is postulated that topical Eflornithine hydrochloride irreversibly inhibits skin ODC activity. This enzyme is necessary in the synthesis of polyamines. Animal data indicate that inhibition of ornithine decarboxylase inhibits cell division and synthetic functions, which affect the rate of hair growth. Eflornithine cream has been shown to retard the rate of hair growth in non-clinical and clinical studies.
Pharmacokinetics
The mean percutaneous absorption of Generic Vaniqa in women with unwanted facial hair, from a 13.9% w/w cream formulation, was <1% of the radioactive dose, following either single or multiple doses under conditions of clinical use, that included shaving within 2 hours before radiolabeled dose application in addition to other forms of cutting or plucking and tweezing to remove facial hair. Steady state was reached within four days of twice-daily application. The apparent steady-state plasma t1/2 of Eflornithine was approximately 8 hours. Following twice daily application of 0.5 g of the cream (total dose 1.0 g/day; 139 mg as anhydrous Eflornithine hydrochloride), under conditions of clinical use in women with unwanted facial hair (n=10), the steady-state Cmax, Ctrough and AUC12hr, were approximately 10 ng/mL, 5 ng/mL, and 92 ng hr/mL, respectively, expressed in terms of the anhydrous free base of Eflornithine hydrochloride. At steady state, the dose-normalized peak concentrations (Cmax) and the extent of daily systemic exposure (AUC) of Eflornithine following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day) is estimated to be approximately 100- and 60-fold lower, respectively, when compared to 370 mg/day once-daily oral doses. This compound is not known to be metabolized and is primarily excreted unchanged in the urine.
INCOMPATIBILITIES
Not applicable
SHELFLIFE
24 Months
PACKAGING INFORMATION
15g Tube
Keep out of reach of children.
STORAGE AND HANDLING INSTRUCTIONS
Store below 30°C. Do not Freeze.
REFERENCES
http://www.ac cessdata.fda.gov/drugsatfda_docs/label/2000/21145lbl.pdf as accessed on 18/5/2015.
Medical Information Compiled in May, 2015
Manufactured in India by:
sun pharmaceutical Ind. ltd.
PLOT NO. B-2, MADKAI INDUSTRIAL
ESTATE, PONDA, GOA - 403 404









