No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
Elidel cream 1% 15g by Novartis
New
Elidel Pimecrolimus cream 15g 1% is used for the treatment of skin disorders such as atopic dermatitis or eczema that affects people aged 2 years and older who have normal immune systems. Eczema is a form of an allergic reaction that causes the skin to be swollen, irritated, red, or itchy.
194 Items
More info


|
Basic Information
|
 |
Are you still troubled by atopic dermatitis and eczema?
Elidel is a non-hormonal anti-inflammatory cream that effectively treats atopic dermatitis and eczema. Pimecrolimus is the active ingredient of Elidel that works by inhibiting the production and release of inflammatory mediators: cytokines in mast cells and T-lymphocytes. This leads to a potent anti-inflammatory effect. The effectiveness of Elidel cream is compared to steroid products. But unlike steroid products, Elidel does not have systemic side effects. 
Elidel- your smart solution for skin problems!
Elidel (Pimecrolimus) cream is a second-line treatment for atopic dermatitis and eczema. The cream possesses a potent anti-inflammatory action characterized by reduction of itching, pain, redness, tiny bumps, or thickening of the skin.
It is used to treat mild to moderate types of diseases both in adults and children. It is especially useful when steroid creams are contraindicated for use. The main advantage of Elidel is a local (on the skin) selective anti-inflammatory effect, combined with a minimal systemic effect on the immune response.
The course of treatment for 6 weeks provides a significant reduction in the signs of skin inflammation - redness, excoriation, infiltration, lichenification, and itching. 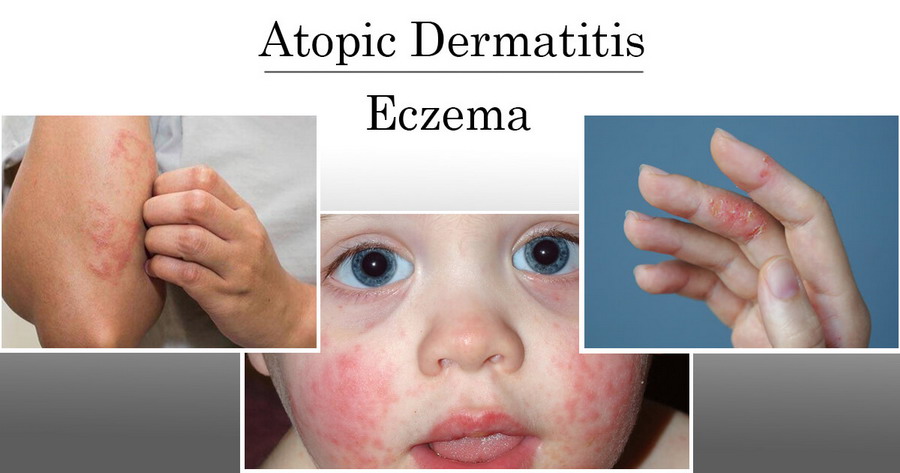
|
Causes of Eczem |
||||
|
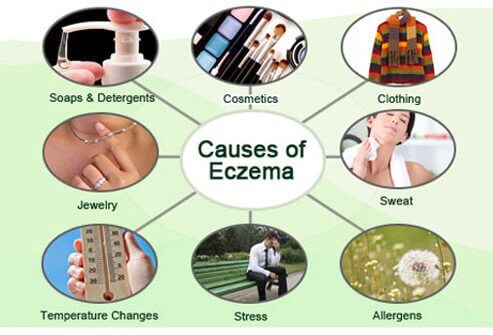
The exact cause of eczema is still unknown. However, it is believed that a combination of triggers and genes play a key role in eczema development.
Eczema triggers include the following: 1.Dry Skin 2.Irritants like metals, soaps, fragrance, formaldehyde 3.Stress 4.Clothing 5.Jewelry 6.Temperature changes 7.Allergens |
 |
|||
|
How to Use Elidel cream |
||||
| Wash and dry your hands. Squeeze cream on your finger and apply a thin layer of Elidel cream on the affected area twice daily (once in the morning and once in the evening). Rub it gently and completely. | If the symptoms persist for more than 6 weeks, the diagnosis of atopic dermatitis needs to be evaluated. | Elidel cream is intended only for external use only. Do not use it in the nose, eyes, and mouth. Do not swallow it. Do not apply it on the skin area affected by the virus (cold sores). | Do not cover the treatment area with an occlusive dressing. | Wash your hands after application. If you use other skincare products, wait until Elidel cream is fully absorbed. |
Elidel cream treatment progress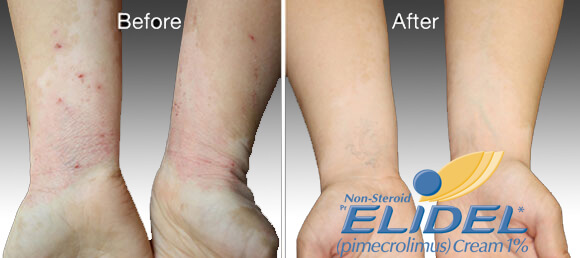
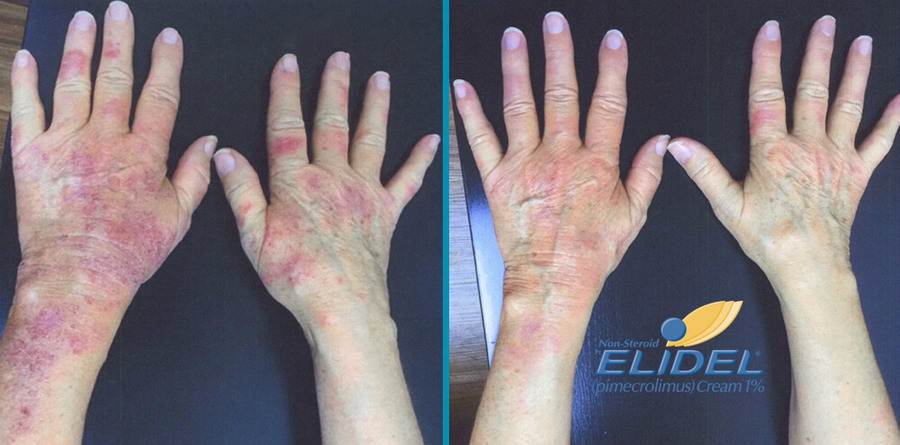
Elidel cream is clinically proven to improve skin condition in patients with atopic dermatitis and eczema.
You should expect to see the first improvement within a week or two. If there is no improvement within 3-6 weeks, consultation of a dermatologist is required.
In most cases, a significant treatment effect is achieved within 15-20 days.
Many skin reactions like erythema, infiltration, excoriations were reduced within 7-8 days.
Full satisfaction is guaranteed!!!
Elidel cream brief description
Product name - Elidel 1 % cream (Pimecrolimus) by Novartis
Qualitative and quantitative composition - Each gram of Elidel cream contains 15 mg (1%) of pimecrolimus. Nonmedicinal ingredients: triglycerides, stearyl alcohol, sodium hydroxide, sodium cetostearyl sulfate, propylene glycol, oleic alcohol, monoglycerides and diglycerides, citric acid, cetyl alcohol, benzyl alcohol, and water.
Action mechanism - Pimecrolimus is an immunosuppressant. It works by decreasing your body's immune system to help slow down the growth of atopic dermatitis (eczema) on your skin. Pimecrolimus can reduce blood cells that help your body fight infections. It is a type of topical calcineurin inhibitor.
Indication - The cream is indicated to eczema patients (atopic dermatitis) who are unable to use or have reacted to other medicines for eczema, such as topical steroids.
Mode of administration - Elidel 1 % cream is applied as a thin layer to the area of the affected skin, twice daily (at approximately 12 hours interval). This medicine can be applied to all affected areas, including the skin on your face and neck.
Contraindication - This medicine should not be applied to infected skin (eg, chickenpox, cold sores, shingles, and bacterial infection). People who develop skin infections while using this medication should contact their doctor immediately.
This medication should not be used by people with Netherton's disease (a rare hereditary skin condition).
Avoid exposing yourself to sunlight, natural or artificial, while using this medication. This medication should not come into contact with the eyes.
Special cautions and warnings - Pimecrolimus cream should not be used in the following circumstances:
-
When you are allergic to pimecrolimus or to any of the ingredients of the medication
-
For children under 2 years old (the effects of this medicine on the developing immune system are unknown)
-
If you have impaired immune function (eg due to AIDS or anti-rejection drugs)
-
In case you have a skin infection in the area to be treated
-
It should never be used at the time of pregnancy unless the advantages are greater than the risks associated. If pregnancy occurs while you use this medicine, contact your doctor immediately.
-
It is not known if pimecrolimus passes into breast milk. If you take this medicine while you are breastfeeding, your baby may feel the effects.
This medication usually starts to provide relief from the symptoms of eczema within a week of starting treatment. If you do not notice a reduction in symptoms after 3 weeks of use, or if you feel that your eczema is getting worse, you should discontinue this medicine and contact your doctor.
Drug interaction - The pimecrolimus can interact with many common drugs. The list includes:
-
Zyrtec (cetirizine)
-
Vitamin D3 (cholecalciferol)
-
triamcinolone topical
-
trazodone
-
tramadol
-
Singulair (montelukast)
-
Protopic (tacrolimus topical)
-
ProAir HFA (albuterol)
-
prednisone
-
omeprazole
-
mupirocin topical
-
metformin
-
levothyroxine
-
hydroxyzine
-
gabapentin
-
Eucrisa (crisaborole topical)
-
doxycycline
-
Cymbalta (duloxetine)
-
cyclobenzaprine
-
clonazepam
-
clobetasol topical
-
Albuterol
So, share with your doctors about the herbal supplements and other medicines you are having when you are prescribed this medicine.
Side effects - Common side effects of Elidel cream includes:
-
Slight itching or redness of the skin.
-
Spots on the skin;
-
Feeling of cooking or heat at the application site (usually mild to moderate and transient or intense and persistent for more than 1 week).
Some of the less common side effects are:
-
Warts
-
Symptoms similar to influenza (runny nose, chest congestion, cough, fever)
-
Rash or hives
-
Herpetic skin infections (cold sores, chickenpox, shingles)
-
Headache (when used continuously for a long time)
-
Discoloration of the skin
-
An infection of the nose and throat
In rare cases, signs of a serious allergic reaction (abdominal cramps, breathing difficulties, nausea, and vomiting, or swelling of the face and throat) may occur.
Interesting facts
-
Pimecrolimus should not be used continuously over a long period of time.
-
Stop using the cream once the symptoms of dermatitis are gone unless advised by your doctor.
-
Avoid contact with eyes. If your hands are not treated, be sure to wash them with soap and water after application of this medicine.
-
Do not cover treated areas with bandages, dressings or wraps (use bandages that allow passage of air)
-
Refrain from taking a bath, shower, or swimming immediately after applying this medication as water may remove the medication.
Elidel Rial Shots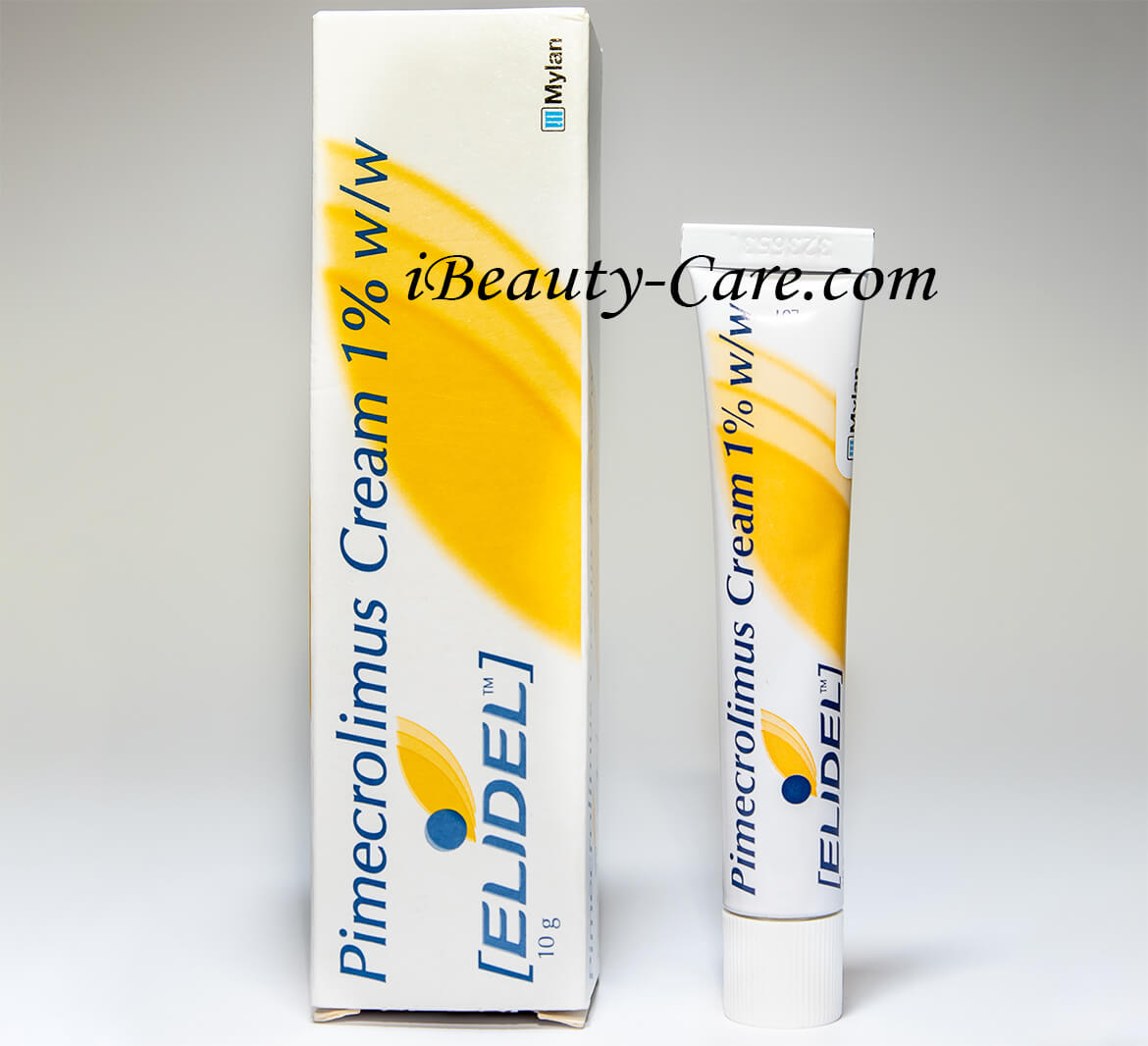



Full Original Product Annotation
PIMECROLIMUS CREAM 1% w/w
(ELIDEL™ 1% CREAM)
Nonsteroid anti-inflammatory dermatological cream for cutaneous use.
COMPOSITION AND PHARMACEUTICAL FORM
SPECIAL WARNINGS AND PRECAUTIONS FOR USE
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
- Data in animals
- Data in humans
- Absorption in adults
- Absorption in children
- Comparison to oral PK Data
- Distribution
- Metabolism
- Elimination
INSTRUCTIONS FOR USE AND HANDLING
COMPOSITION AND PHARMACEUTICAL FORM
Each gram contains 10 mg Pimecrolimus.
For a full list of excipients, see section EXCIPIENTS.
External use. The cream is whitish, odourless, non-staining, and easily spreadable.
Atopic dermatitis (eczema) (AD).
Elidel cream 1% is indicated for the treatment of mild to moderate atopic dermatitis in patients 3 months of age and above.
DOSAGE AND ADMINISTRATION
As directed by the Physician
Apply a thin layer of Elidel cream 1% to the affected skin twice daily and rub in gently and completely.
Elidel cream 1% may be used on all skin areas, including the head and face, neck and intertriginous areas.
In the long-term management of atopic dermatitis (eczema), Elidel cream 1% treatment should begin at first appearance of signs and symptoms of atopic dermatitis to prevent flares of the disease. Elidel cream 1% should be used twice daily until signs and symptoms resolve. If signs and symptoms persist beyond 6 weeks, patients should be re-examined to confirm the diagnosis of atopic dermatitis. If discontinued, treatment should be resumed upon first recurrence of signs and symptoms to prevent flares of the disease.
Emollients can be applied immediately after using Elidel cream 1%. However, after a bath/shower, emollients should be applied before using Elidel cream 1%.
Continuous long-term use of ELIDEL Cream should be avoided, and application should be limited to areas of involvement with atopic dermatitis.
For infants (3 to 23 months), children (2 to 11 years), and adolescents (12 to 17 years) the dosing recommendation is the same as for adults.
Use in babies under 3 months of age has not been evaluated.
Atopic dermatitis (eczema) is rarely observed in patients aged 65 and over. Clinical studies with Elidel cream 1% did not include a sufficient number of patients in this age range to determine whether they respond differently from younger patients.
CONTRAINDICATIONS
Known hypersensitivity to pimecrolimus or to any of the excipients (see section EXCIPIENTS).
SPECIAL WARNINGS AND PRECAUTIONS FOR USE
Long-term safety of Elidel cream 1% has not been established.
Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including Elidel cream 1% (see section UNDESIRABLE EFFECTS).
As the long-term effect on the local skin immune response and on the incidence of skin malignancies is unknown, Elidel cream 1% should not be applied to potentially malignant or pre-malignant skin lesions.
Elidel cream 1% should not be applied to areas affected by acute cutaneous viral infections.
In the presence of a dermatological bacterial or fungal infection, the use of an appropriate antimicrobial agent should be instituted. If resolution of the infection does not occur, Elidel cream 1% should be discontinued until the infection has been adequately controlled. The safety of Elidel cream 1% has not been established in patients with Netherton’s Syndrome and generalised erythroderma. Elidel cream 1% is not recommended in patients with Netherton’s syndrome or severely inflamed or damaged skin (e.g. erythroderma) where there is a potential for increased absorption.
The safety and efficacy of Elidel cream 1% in immunocompromised patients have not been studied. The use in immunocompromised patients is therefore not recommended.
In clinical studies, 14/1,544 (0.9%) cases of lymphadenopathy were reported while using Elidel cream 1%. These cases of lymphadenopathy were usually related to infections and noted to resolve upon appropriate antibiotic therapy. Of these 14 cases, the majority had either a clear etiology or were known to resolve. Patients who receive Elidel cream 1% and who develop lymphadenopathy should have the etiology of their lymphadenopathy investigated. In the absence of a clear etiology for the lymphadenopathy, or in the presence of acute infectious mononucleosis, Elidel cream 1% should be discontinued. Patients who develop lymphadenopathy should be monitored to ensure that the lymphadenopathy resolves.
Throughout the course of treatment, it is prudent for patients to minimize or avoid natural or artificial sunlight exposure. The potential effects of Elidel cream 1% on skin response to ultraviolet damage are not known (see section PRECLINI CAL SAFETY DATA).
Use of Elidel cream 1% may cause mild and transient reactions at the site of application, such as a feeling of warmth and/or burning sensation. Patients should see a physician if an application site reaction is severe.
Care should be taken to avoid contact with eyes and mucous membranes. If accidentally applied to these areas, the cream should be thoroughly wiped off and rinsed off with water.
INTERACTIONS
Potential interactions between Elidel cream 1% and other drugs have not been systematically evaluated. Based on its minimal extent of absorption, interactions of Elidel cream 1% with systemically administered drugs are unlikely to occur (see section PHARMACOKINETICS).
A study that included 79 infants treated for up to 2 years showed that treatment with Elidel cream 1% did not interfere with the protective immune response to childhood vaccinations. Application of Elidel cream 1% to vaccination sites, as long as local reactions persist, was not studied and is therefore not recommended.
PREGNANCY AND LACTATION
Pregnancy
There are no adequate data from the use of Elidel cream 1% in pregnant women. Animal studies using dermal application do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/fetal development, parturition or postnatal development (see section PRECLINICAL SAFETY DATA).
Caution should be exercised when prescribing Elidel cream 1% to pregnant women. However, based on the minimal extent of pimecrolimus absorption after topical application of Elidel cream 1% (see section PHARMACOKINETICS), the potential risk for humans is considered limited.
Lactation
Animal studies on milk excretion after topical application were not conducted. It is not known whether pimecrolimus is excreted in the milk after topical application. Because many drugs are excreted in human milk, caution should be exercised when Elidel cream 1% is administered to a nursing woman. However, based on the minimal extent of pimecrolimus absorption after topical application of Elidel cream 1%, (see section PHARMACOKINETICS), the potential risk for humans is considered limited.
Nursing mothers should not apply Elidel cream 1% to the breast.
Fertility
There are no clinical data on the effects of pimecrolimus on male or female fertility (see section PRECLINI CAL SAFETY DATA).
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
Elidel cream 1% has no known effect on the ability to drive and use machines.
UNDESIRABLE EFFECTS
The safety profile of Elidel cream 1% has been established in more than 2000 patients including infants (>= 3 months), children, adolescents, and adults enrolled in phase 2 and 3 studies. Over 1500 of these patients were treated with Elidel cream 1% and over 500 were treated with control treatment i.e. either Elidel vehicle and/or topical corticosteroids.
The most common adverse events were application site reactions which were reported by approximately 19% of the patients treated with Elidel cream 1% and 16% of patients in the control group. These reactions generally occurred early in treatment, were mild/ moderate in severity and were of short duration.
Adverse reactions (Table 1) are ranked under heading of frequency, the most frequent first, using the following convention: very common (>= 1/10); common (>= 1/100, < 1/10); uncommon (>= 1/1,000, < 1/100); rare (>= 1/10,000, < 1/1,000); very rare (< 1/10,000), including isolated reports.
Table 1
|
Skin and subcutaneous tissue disorders |
|
|
Very common |
application site burning |
|
Common |
application site reactions (irritation, pruritus and erythema), skin infections (folliculitis) |
|
Uncommon |
impetigo, condition aggravated, herpes simplex, herpes simplex dermatitis (eczema herpeticum), molluscum contagiosum, application site disorders such as rash, pain, paraesthesia, desquamation, dryness, oedema, skin papilloma, furuncle |
The following adverse reactions have been reported during post-marketing experience. The frequency has been estimated from the reporting rates. Because these reactions are reported voluntarily from a population of uncertain size, the frequency reflects only an estimate.
|
Immune system disorders |
|
|
Very rare |
anaphylactic reactions |
|
Metabolism and nutrition disorders |
|
|
Rare |
alcohol intolerance1 |
|
Skin and subcutaneous tissue disorders |
|
|
Rare |
allergic reactions (e.g. rash, urticaria, angioedema), skin discoloration (e.g. hypopigmentation, hyperpigmentation) |
1In most cases, flushing, rash, burning, itching or swelling occurred shortly after the intake of alcohol.
Rare cases of malignancy, including cutaneous and other types of lymphoma, and skin cancers, have been reported in patients using pimecrolimus cream, although no causal relationship has been established (see section SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
There has been no experience of overdose with Elidel cream 1%.
PHARMACODYNAMICS
Non-clinical pharmacology
Pimecrolimus is an anti-inflammatory ascomycin macrolactam derivative and a selective inhibitor of the production and release of pro-inflammatory cytokines and mediators in T cells and mast cells.
Pimecrolimus binds with high affinity to macrophilin-12 and inhibits the calcium-dependent phosphatase calcineurin. As a consequence, it inhibits T cell proliferation and prevents the transcription and release of both T helper type 1 cell (TH1) and T helper type 2 cell (TH2) inflammatory cytokines such as interleukin-2, interferon-gamma, interleukin-4, interleukin-5, interleukin-10, tumor necrosis factor alpha and granulocyte macrophage colony-stimulating factor. Pimecrolimus and tacrolimus have similar potencies to inhibit recall antigen responses in human T-helper cell clones, isolated from the skin of an atopic dermatitis patient. Pimecrolimus also prevents the release of cytokines and pro-inflammatory mediators from mast cells in vitro after stimulation by antigen/lgE. Pimecrolimus does not affect the growth of keratinocyte, fibroblast or endothelial cell lines and, in contrast to corticosteroids, does not impair the differentiation, maturation, functions and viability of murine Langerhans cells and human monocytes-derived dendritic cells, thus, underlining its cell-selective mode of action.
In studies using various topical formulations, including the pimecrolimus cream and tacrolimus ointment, pimecrolimus penetrates similarly into, but permeates less through skin in vitro than corticosteroids or tacrolimus, suggesting a lower systemic exposure to pimecrolimus after topical application as compared to tacrolimus and corticosteroids.
Pimecrolimus exhibits high anti-inflammatory activity in animal models of skin inflammation after topical and systemic application. Pimecrolimus is as effective as the high potency corticosteroids clobetasol-17-propionate and fluticasone after topical application in the pig model of allergic contact dermatitis (ACD). Topical pimecrolimus also inhibits the inflammatory response to irritants, as shown in murine models of irritant contact dermatitis. Furthermore, topical and oral pimecrolimus effectively reduces skin inflammation and pruritus and normalises histopathological changes in hypomagnesemic hairless rats, a model that mimics acute aspects of atopic dermatitis. Oral pimecrolimus is superior to ciclosporin A by a factor of 4 and superior to tacrolimus by a factor of more than 2 in inhibiting skin inflammation in ACD of rats.
Topical pimecrolimus does not cause skin atrophy in pigs, unlike clobetasol-17-propionate. Furthermore, pimecrolimus does not cause blanching and changes in skin texture in pigs, unlike clobetasol-17-propionate and fluticasone.
Topical pimecrolimus does not affect epidermal Langerhans' cells in mice. In contrast, treatment with standard topical corticosteroids, including hydrocortisone, resulted in a reduction in Langerhans cells by 96 to 100%. A recent analysis of skin biopsies of atopic dermatitis patients has confirmed that treatment with the corticosteroid beta-methasone 0.1%, but not Elidel cream 1%, for 3 weeks results in depletion of Langerhans cells, while both drugs significantly reduce T cells. Thus, results from these as well as in vitro studies indicate that topically applied pimecrolimus is unlikely to interfere with the function of Langerhans/dendritic cells to differentiate naive T cells into effector T cells, which is key for the developing immune system and maintenance of specific immunocompetence. In contrast to its efficacy in skin inflammation models, the potential of pimecrolimus for affecting systemic immune responses is lower than that of tacrolimus and ciclosporin A, as shown in models of systemic immunosuppression and based on dose comparison. In the rat, after subcutaneous administration, the potency of pimecrolimus in inhibiting the formation of antibodies is 48-fold lower than with tacrolimus. Subcutaneous injections of ciclosporin A and tacrolimus suppress the localized graft-versus-host reaction in rats 8-fold and 66-fold more potently than pimecrolimus. In contrast to ciclosporin A and tacrolimus, oral treatment of mice with pimecrolimus neither impairs the primary immune response nor decreases lymph node weight and cellularity in ACD.
The data show that topical pimecrolimus/ Elidel has a high and selective anti-inflammatory activity in the skin and minimal percutaneous resorption. It differs from corticosteroids by its selective action on T cells and mast cells, by lack of impairment of Langerhans’ cells/dendritic cells, by lack of induction of skin atrophy and by less permeation through skin. It differs from tacrolimus by less permeation through skin and by a lower potential for affecting systemic immune responses.
In animal safety pharmacology studies, single oral doses of pimecrolimus had no effect on basal lung and cardiovascular functions. CNS and endocrine parameters (e.g. GH, prolactin, LH, testosterone, corticosterone) were also unaffected. Based on its mechanism of action as a selective inhibitor of the production and release of pro-inflammatory cytokines and mediators in T cells and mast cells, pimecrolimus is not expected to have any effect on the HPA axis.
Clinical data
Short-term (acute) treatment in paediatric patients
Children and adolescents: Two 6-week, vehicle-controlled trials were conducted including a total of 403 paediatric patients aged 2 to 17 years. Patients were treated twice daily with Elidel cream 1%. The data of both studies were pooled.
Infants: A similar 6-week study was conducted in 186 patients aged 3 to 23 months.
In these three 6-week studies, the efficacy results at endpoint were as follows:
|
|
|
Children and adolescents |
Infants |
||||
|
Endpoint |
Criteria |
Elidel 1% (N=267) |
Vehicle (N=136) |
p-value |
Elidel 1% (N=123) |
Vehicle (N=63) |
p-value |
|
IGA*: |
Clear or almost clear1 |
34.8% |
18.4% |
< 0.001 |
54.5% |
23.8% |
<0.001 |
|
IGA*: |
Improvement2 |
59.9% |
33% |
not done |
68% |
40% |
Not done |
|
Pruritus: |
Absent or mild |
56.6% |
33.8% |
< 0.001 |
72.4% |
33.3% |
<0.001 |
|
EASI°: |
Overall (mean% change)3 |
-43.6 |
-0.7 |
< 0.001 |
-61.8 |
+ 7.35 |
<0.001 |
|
EASI°: |
Head/Neck (mean% change)3 |
-61.1 |
+ 0.6 |
< 0.001 |
-74.0 |
+ 31.48 |
<0.001 |
|
* Investigators Global Assessment ° Eczema Area Severity Index (EASI): mean% change in clinical signs (erythema, infiltration, excoriation, lichenification) and body surface area involved 1p-value based on CMH test stratified by centre 3lmprovement=lower IGA than at baseline 3p-value based on ANCOVA model of EASI at Day 43 endpoint, with centre and treatment as factors and baseline (Day 1) EASI a covariate |
|||||||
A significant improvement in pruritus was observed within the first week of treatment in 44% of children and adolescents and in 70% of infants.
Long-term treatment in paediatric patients
In two double-blind studies of long-term management of atopic dermatitis in 713 children and adolescents (2 to 17 years) and 251 infants (3 to 23 months), Elidel cream 1% was evaluated as first line foundation therapy.
In addition to emollients, the Elidel group received Elidel cream 1% used at first signs of itching and redness to prevent progression to flares of atopic dermatitis. Only in case of flare not controlled by Elidel cream 1%, treatment with medium potency topical corticosteroids was initiated.
The control group received a standard treatment consisting of emollient plus medium potency topical corticosteroids to treat flares. Elidel vehicle was used instead of Elidel cream 1% in order to maintain the studies blind.
Both studies showed a reduction in the incidence of flares (p < 0.001) in favour of Elidel cream 1% first-line treatment; Elidel cream 1% first-line treatment showed better efficacy in all secondary assessments (Eczema Area Severity Index, IGA, subject assessment); pruritus was controlled within a week with Elidel cream 1%. Significantly more patients on Elidel cream 1% completed 6 months (children (61% Elidel cream 1% vs 34% control); infants (70% Elidel vs 33% control) and 12 months (children 51% Elidel vs 28% control) with no flare. Significantly more patients treated with Elidel cream 1% did not use corticosteroids in the first 6 months (children: 65% Elidel vs 37% control; infants: 70% Elidel vs 39% control) or 12 months (children: 57% Elidel cream 1% vs 32% control). The efficacy of Elidel cream 1% was maintained over time with the ability to prevent disease progression to severe flares.
Special studies
Tolerability studies demonstrated that Elidel cream 1% is devoid of any irritation, contact sensitising, phototoxic or photosensitising potential.
The atrophogenic potential of Elidel cream 1% in humans was tested in comparison to medium and highly potent topical steroids (betamethasone-17-valerate 0.1% cream, triamcinolone acetonide 0.1% cream) and vehicle in sixteen healthy volunteers treated for 4 weeks. Both topical corticosteroids, induced a significant reduction in skin thickness measured by echography, as compared to Elidel cream 1% and vehicle, which did not induce a reduction of skin thickness.
PHARMACOKINETICS
Data in animals
Pimecrolimus is lipophilic. When applied topically its permeation through skin is very low. In minipigs, the total drug-related material systemically absorbed following a single 22 h application of Elidel cream 1% under semi-occlusion was at most 1% of the dose; the bioavailability of unchanged pimecrolimus was estimated to be about 0.03%. The amount of radiolabeled drug-related material in the skin at the application site remained essentially constant in the time interval Oto 10 days after a 22-hour application; at 5 days postdose, it represented almost exclusively unchanged pimecrolimus. The major fraction of the absorbed topical dose was completely metabolised and excreted slowly via the bile into the faeces.
Data in humans
Absorption in adults
Systemic exposure to pimecrolimus was investigated in 12 adult patients treated with Elidel cream 1% twice daily for 3 weeks. These patients had atopic dermatitis (eczema) lesions affecting 15 to 59% of their body surface area (BSA). 77.5% of pimecrolimus blood concentrations were below 0.5 ng/mL, the assay limit of quantitation (LoQ), and 99.8% of the total samples were below 1 ng/mL. The highest blood concentration of pimecrolimus measured in one patient was 1.4 ng/mL.
In 40 adult patients treated for up to 1 year with Elidel, having 14 to 62% of their BSA affected at baseline, 98% of pimecrolimus blood concentrations of pimecrolimus were consistently low, mostly below the LoQ. A maximum blood concentration of 0.8 ng/mL was measured in only 2 patients in week 6 of treatment. There was no increase in blood concentration over time in any patient during the 12 months of treatment. In 13 adult patients with hand dermatitis treated with Elidel twice daily for 3 weeks (palmar and dorsal surfaces of hands treated, overnight occlusion), the maximum blood concentration of pimecrolimus measured was 0.91 ng/mL.
Given the high proportion of pimecrolimus blood levels below the LoQ after topical application, the AUG could only be calculated from a few individuals. In 8 adult AD patients presenting with at least three quantifiable blood levels per visit day, the AUC(0to12h) од values ranged from 2.5 to 11.4 ng * h/mL.
Absorption in children
Systemic exposure to pimecrolimus was investigated in 58 paediatric patients aged 3 months to 14 years, who had atopic dermatitis (eczema) lesions involving 10 to 92% of the total body surface area. These children were treated with Elidel cream 1% twice daily for 3 weeks and five out of them were treated for up to 1 year on a “as needed” basis.
The blood concentrations measured in these paediatric patients were consistently low regardless of the extent of lesions treated or duration of therapy. They were in a range similar to that measured in adult patients treated under the same dosing regimen. 60% of pimecrolimus blood concentrations were below 0.5 ng/mL (LoQ) and 97% of all samples were below 2 ng/mL. The highest blood concentrations measured in 2 paediatric patients aged 8 months to 14 years of age were 2.0 ng/mL.
In the youngest patients (aged 3 to 23 months), the highest blood concentration measured in one patient was 2.6 ng/mL. In the 5 children treated for 1 year, blood concentrations were consistently low, and the maximum blood concentration measured was 1.94 ng/mL (1 patient). In these five patients, there was no increase in blood concentration over time in any patient during the 12 months of treatment. In 8 paediatric patients aged 2 to 14 years presenting at least three measurable blood concentrations per visit day, AUC(0to12h) ranged from 5.4 to 18.8 ng * h/mL. AUG ranges observed in patients with < 40% BSA affected at baseline were comparable to those in patients with >=40% BSA.
Comparison to oral PK Data
In psoriatic patients treated with oral pimecrolimus doses ranging from 5 mg once daily to 30 mg twice daily for 4 weeks, the drug was well tolerated at all doses including the highest dose. No significant adverse events were reported and no significant change was observed in physical examination, vital signs, and laboratory (including renal) safety parameters. The highest dose was associated with an AUC(0to12h) of 294.9 ng x h/mL. This exposure is approximately 26 and 16 times higher, respectively, then the highest systemic exposure observed in adult and paediatric atopic dermatitis (eczema) patients treated topically with Elidel twice daily for 3 weeks (AUC(0to12h) of 11.4 ng *h/mL and 18.8 ng * h/mL, respectively).
Distribution
Consistent with its skin selectivity, after topical application, pimecrolimus blood levels are very low. Therefore, pimecrolimus metabolism could not be determined after topical administration.
In vitro plasma protein binding studies have shown that 99.6% of pimecrolimus in plasma is bound to proteins. The major fraction of pimecrolimus in plasma is bound to different lipoproteins.
Metabolism
After single oral administration of radiolabeled pimecrolimus in healthy subjects, unchanged pimecrolimus was the major drug-related component in blood and there were numerous minor metabolites of moderate polarity that appeared to be products of O-demethylations and oxygenation.
No drug metabolism was observed in human skin in vitro.
Elimination
Drug-related radioactivity was excreted principally via the faeces (78.4%) and only a small fraction (2.5%) was recovered in urine. Total mean recovery of radioactivity was 80.9%. Parent compound was not detected in urine and less than 1% of radioactivity in faeces was accounted for by unchanged pimecrolimus.
PRECLINICAL SAFETY DATA
Toxicology studies after dermal application
A variety of preclinical safety studies were conducted with the pimecrolimus cream formulations in several animal species. There was no evidence of irritation, (photo) sensitisation, or local or systemic toxicity.
In a 2-year dermal carcinogenicity study in rats using Elidel cream 1%, no cutaneous or systemic carcinogenic effects were observed up to the highest practicable dose of 10 mg/kg/day or 110 mg/m-/day, represented by a mean AUC(0 to 24) of 125 ng * h/mL (equivalent to 3.3 times the maximum exposure observed in paediatric patients in clinical trials). In a mouse dermal carcinogenicity study using pimecrolimus in an ethanolic solution, no increase in incidence of neoplasms was observed in the skin or other organs up to the highest dose of 4 mg/kg/day or 12 mg/m2/day, corresponding to a mean AUC(0 to 24) value of 1,040 ng x h/mL (equivalent to 27 times the maximum exposure observed in paediatric patients in clinical trials).
In a dermal photocarcinogenicity study in hairless mice using Elidel cream, no photocarcinogenic effect versus vehicle treated animals was noted up to the highest dose of 10 mg/kg/day or 30 mg/m2/day, corresponding to a mean AUC(0 to 24) value of 2,100 ng x h/mL (equivalent to 55 times the maximum exposure observed in pediatric patients in clinical trials).
In dermal reproduction studies, no maternal or fetal toxicity was observed up to the highest practicable doses tested. 10 mg/kg/day or 110 mg/m2/day in rats and 10 mg/kg/day or 36 mg/m2/day in rabbits. In rabbits, the corresponding mean AUC(0 to 24) was 24.8 ng*h/mL. AUG could not be calculated in rats.
Toxicology studies after oral administration
Adverse reactions not observed in clinical studies but seen in animals at exposures considered sufficiently in excess of the maximum human exposure, indicating little relevance to clinical use, were as follows: reproduction studies in rats receiving oral doses up to 45 mg/kg/day or 490 mg/m2/day, corresponding to an extrapolated mean AUC(0 to 24) of 1448 ng * h/mL (equivalent to at least 63 times the maximum exposure observed in adult patients), revealed slight maternal toxicity, oestrus cycle disturbances, postimplantation loss and reduction in litter size.
An oral fertility and embryo-foetal developmental study in rats revealed estrus cycle disturbances, post-implantation loss and reduction in litter size at the 45 mg/kg/day dose (38 times the Maximum Recommended Human Dose (MRHD) based on AUG comparisons).
No effect on fertility in female rats was noted at 10 mg/kg/day (12 x MRHD based on AUG comparisons). No effect on fertility in male rats was noted at 45 mg/kg/day (23 x MRHD based on AUG comparisons), which was the highest dose tested in this study.
A second oral fertility and embryo-foetal developmental study in rats revealed reduced testicular and epididymal weights, reduced testicular sperm counts and motile sperm for males and estrus cycle disturbances, decreased corpora lutea, decreased implantations and viable fetuses for females at 45 mg/kg/day dose (123 x MRHD for males and 192 x MRHD for females based on AUG comparisons). No effect on fertility in female rats was noted at 10 mg/kg/day (5 x MRHD based on AUG comparisons). No effect on fertility in male rats was noted at 2 mg/kg/day (0.7 x MRHD based on AUG comparisons).
In an oral reproduction study in rabbits, maternal toxicity, but no embryotoxicity or teratogenicity was observed at the highest dose of 20 mg/kg/day or 72 mg/m2/day corresponding to an extrapolated mean AUC(0 to 24) value of 147 ng x h/mL (equivalent to at least 6 times the maximum exposure observed in adult patients).
In a mouse oral carcinogenicity study, a 13% higher incidence of lymphomas versus controls associated with signs of immunosuppression was observed at 45 mg/kg/day or 135 mg/m2/day, corresponding to a mean AUC(0 to 24) value of 9,821 ng x h/mL (equivalent to at least 258 times the maximum exposure observed in paediatric patients in clinical trials), A dose of 15 mg/kg/day or 45 mg/m2/day, corresponding to a mean AUC(0 to 24) value of 5,059 ng x h/mL, produced no lymphomas or discernible effects on the immune system (equivalent to 133 times the maximum exposure observed in paediatric patients in clinical trials). In an oral rat carcinogenicity study, no carcinogenic potential was observed up to a dose of 10 mg/kg/day or 110 mg/m2/day, exceeding the maximum tolerated dose, represented by a mean AUC(0 to 24) value of 1,550 ng* h/mL (equivalent to 41 times the maximum exposure observed in pediatric patients in clinical trials).
In a 39-week monkey oral toxicity study, a dose-related immunosuppressive-related lymphoproliferative disorder (IRLD) associated with iymphocryptovirus (LCV) and other opportunistic infections were observed, beginning at 15 mg/kg/day, corresponding to a mean AUC(0 to 24) value of 1,193 ngx h/mL(31 times the maximum exposure observed in pediatric patients in clinical trials).
At 45 mg/kg/day, corresponding to a mean AUC(0 to 24) value of 3,945 ng * h/mL (104 times the maximum exposure observed in pediatric patients in clinical trials) IRLD was accompanied by mortality/moribundity, food consumption and body weight reductions, and pathological changes secondary to compound-related immunosuppression. Recovery and/or at least partial reversibility of the effects were noted upon cessation of dosage.
A battery of in vitro and in vivo genotoxicity texts, including the Ames assay, mouse lymphoma L5178Y assay, chromosome aberration test in V79 Chinese hamster cells, and mouse micronucleus test revealed no evidence for a mutagenic or clastogenic potential of the drug.
Triglycerides, oleyl alcohol, propylene glycol, stearyl alcohol, cetyl alcohol, mono-and diglycerides, sodium cetostearyl sulphate, benzyl alcohol, citric acid, sodium hydroxide, purified water.
Pharmaceutical formulations may vary between countries.
INCOMPATIBILITIES
In the absence of compatibility studies, this medicinal product must not be mixed with other topical medicinal products.
Emollients can be applied together with Elidel cream 1% (see section DOSAGE AND ADMINISTRATION).
STORAGE
See folding box.
Elidel should not be used after the date marked "EXP" on the pack.
INSTRUCTIONS FOR USE AND HANDLING
Note: Elidel should be kept out of the reach and sight of children.
Always use Elidel cream 1% exactly as your doctor or pharmacist has instructed you. Please check with your doctor or pharmacist if you have any questions.
You can use Elidel cream 1% on all skin areas, including on the head, face and neck and in the folds of the skin. Use Elidel cream 1% only on areas of your skin that have eczema.
Follow your doctor's advice if symptoms of eczema return after a treatment with Elidel cream 1%.
Apply the cream as follows:
- Wash and dry your hands.
- Open the tube (the first time you use the tube you will need to break the seal using the spike in the top of the cap).
- Squeeze cream onto your finger.
- Apply a thin layer of Elidel cream 1% and completely cover the affected skin.
- Rub in gently and completely
- Replace the cap on the tube.
The cream should be applied twice daily, for instance once in the morning and once in the evening. Moisturisers (emollients) can be applied immediately after using Elidel cream 1%. However, after a bath/shower, moisturisers (emollients) should be applied before using Elidel cream 1%.
If you have not noticed any signs of improvement after 6 weeks of treatment, consult your doctor. Sometimes other skin diseases can look like eczema.
Any unused product or waste material should be disposed of in accordance with local requirements.
Once opened, the contents of the tube should be used within 1 year.
Manufactured by: MEDA Manufacturing, Avenue J. F. Kennedy, 33700 Merignac, France









