No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
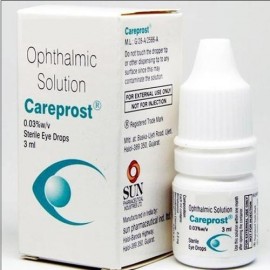
Careprost
New
Сareprost contains the active ingredient Bimatoprost in form of eye drops which are used to reduce elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension. Bimatoprost is an active ingredient in the group of prostaglandin analogs and it widely used with cosmetic purpose to increase eyelashes growth.
979 Items
More info


|
Basic Information
|
 |
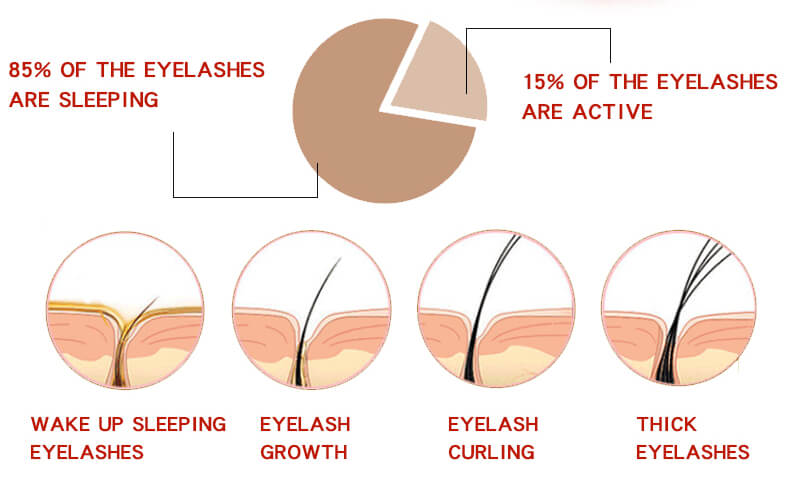
Are you still troubled by these problems?
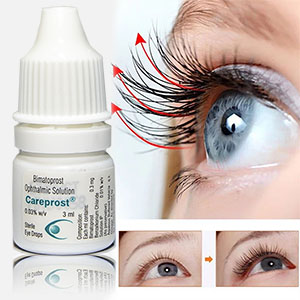
Careprost is a commercial name of Bimatoprost. It is the only available and approved solution that makes eyelashes longer, thicker, and darker. Inadequate or insufficient eyelashes growth is known as a health problem called hypotrichosis.
The good news is that Careprost resolves this problem easily. It focuses on the root of the problem and this makes Careprost a really fantastic and unique product. Forget about brittle and frail lashes with Careprost.
Activate Eyelashes Growth Power
No more false lashes needed. Careprost is the best choice clinically proven to enhance eyelashes growth. Just apply Careprost on a regular basis and get great results in just 2 months. Insufficient, brittle, fragile lashes are no longer a problem. Careprost helps millions of women enjoy thick, long, and dark lashes. 100% satisfaction is guaranteed. 
|
Why do you need to use Careprost? |
||||
|
 |
|||

|
How to Use Careprost |
||||
 |
 |
 |
 |
 |
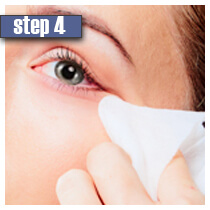
| Make sure your face is washed prior to using Careprost. If you wear contact lenses, they should be removed. Try to apply Careprost solution on a daily basis before bedtime. | Remove a sterile applicator and hold it horizontally. Put only one drop of Careprost solution on the applicator. If too much of Careprost solution is applied, it may get into the eyes and cause irritation. | Draw the applicator along the skin of the eyelid in the place where eyelashes meet the skin. | Use a tissue or cotton to remove an excess of solution. Only use sterile applicators to apply Careprost | Throw the applicator away in the trash. Contact lenses may be installed only after 15 minutes. Do not apply Careprost solution more than once daily. This will not speed up the results. |
Careprost weekly progress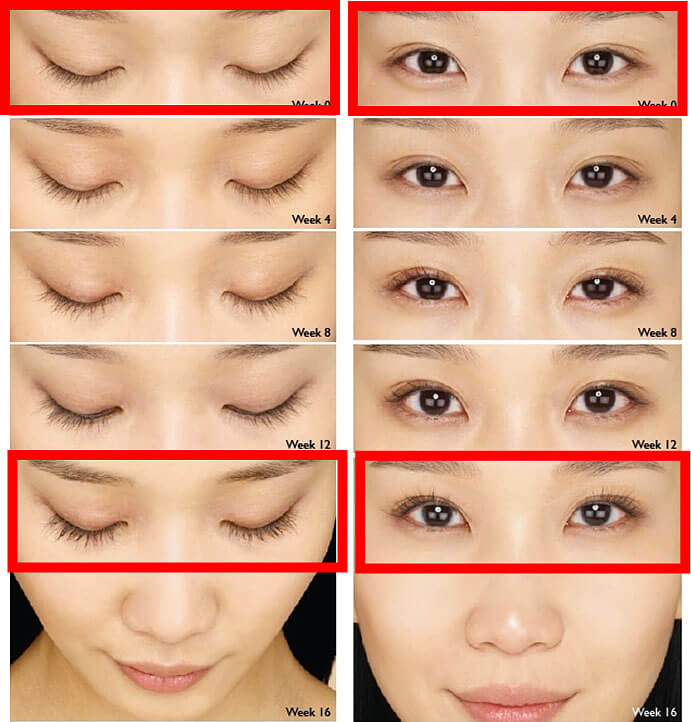
The results may vary depending on many factors such as age, eyelashes condition, and many others. According to the results of clinical studies, first visible results are achieved within 8 weeks.
- 1st week – 5% growth (Careprost). Your start the application and wait for the result. The process begins.
- 4th week – 15% growth and some first results are already visible. Continue using Careprost as recommended.
- 8th week – 50% growth of eyelashes. Lashes become longer, thicker, and darker.
- 12th week – 69% growth with Careprost. The lashes are gorgeous.
- 16th week – 78%-82% growth with Bimatoprost. Lashes look so long.
- 20th week – 82-106% growth with Bimatoprost. Stabilization of lashes growth.
Full satisfaction is guaranteed!!!
Latisse and Careprost are identical products made by two different manufacturers. They contain the same active ingredient Bimatoprost ophtalmic solution 0.03% 3ml and are used for the same purposes. Both products provide great results in eyelashes growth.
- Latisse=Careprost
- Same active ingredients - Bimatoprost
- Same composition
- Same volume
- Same indications
- Same results for less money

Generic Latisse brief description
Product name - Careprost - Bimatoprost ophtalmic solution
Qualitative and quantitative composition - Careprost is an ophthalmic solution containing bimatoprost (C25H37NO4). It contains 0.3mg/ml or 0.1mg/ml bimatoprost.
Action mechanism - Careprost Eye Drop promotes the lowering of intraocular pressure by facilitating fluid secretion that has accumulated in the interior of the eye. The fluid is called aqueous humor and is constantly produced and excreted by your eyes. When the excretion process gets slow, Careprost eye drops help to increase it to the normal level. This balances intraocular pressure and drastically reduces the risk of optic nerve damage.
Bimatoprost, the active ingredient of Careprost induces mild stimulation to flow rate of aqueous humor both at night and day (14% and 13% respectively). The ocular hypotensive property of this component is basically because of the decrease in tonographic resistance (26%) to outflow.
Indication - It is indicated to reduce elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension. It has cosmetic use in increasing eyelash growth/length.
Mode of administration - Careprost eye drops 0.3mg/ml should be given once daily in the affected eye (usually in the evening), according to the instructions of your doctor or pharmacist.
Wearing contact lenses may affect the actions of the Careprost eye drops. So, before using Careprost eye drops, you should remove your contact lenses and replace them only after the drops have begun its action (usually after fifteen minutes).
There is no specific time frame for the use of Careprost eye drops. Each patient responds differently to the treatment and you should follow your doctor's instructions after monitoring your progress. This means that you may need to use the drops for several months or even years. The goal of using Careprost eye drops is to lower the intraocular pressure, but it will not cure glaucoma.
For cosmetic use, it is applied on the base of the eyelashes, directly on the skin. Clean your hand before the use and always use a clean (preferably sterile) applicator.
Contraindication - You should not use Careprost eye drops if any of the following conditions are present:
-
You are allergic to bimatoprost (the main component of Careprost) or any of the ingredients contained in Careprost eye drops
-
During pregnancy or when you have the intention to be pregnant or a lactating woman
-
You have liver, kidney or respiratory diseases
Special cautions and warnings - If you miss taking Careprost eye drops, take them as soon as you remember, as long as it is not the time to take the next dose. Do not take a double dose to replace the missed one. There are no serious effects of an overdose. If you suspect an overdose, you should seek medical advice.
The action of this drug in the ophthalmic form can be seen 4 hours after the dosing. The effect wears off after 12 to 24 hours of dosing.
The cumulative implications of the topical use of an ophthalmic form of this medication can be noted usually within the second month of the initial dose. After discontinuation, the effect may last for the next 3 to 4 weeks.
Drug interaction - If you are taking other medications, whether they are prescribed by a doctor or bought at the pharmacy, the combination of medications may hinder the function of Careprost eye drops. If you are using other eye drops, contact your doctor and tell him/her the names of the medications you are using before starting the Careprost eye drops. Careprost eye drops may interact with latanoprost.
Side effects - The most commonly reported side effects with using Careprost Eye Drops 0.3mg are:
Reddened and clogged eyes (conjunctival hyperemia), itching, irritation or inflammation of the eye (conjunctivitis) and eyelid (eyelid firing), photosensitivity, increase in pigmentation of the eye or area around the eye, optical changes, eyelash darkening, iris dimming, headache, and dizziness. If you have any of these side effects daily, tell your doctor immediately.
Interesting facts
-
Careprost eye drops should be stored in a cool place, away from moisture, where the temperature is below 25℃
-
Dispose of the bottle four weeks after opening it
-
Careprost has no interaction with alcohol
-
It has no serious effect on kidney or liver
-
It may affect your lungs in rare cases
-
The drug has no tendencies of forming a habit
The charm of your eyes depends on your eyelashes
It is your turn to try Bimat and enjoy your natural eyelashes. Your wish comes true with Latisse generic.
We guarantee that you won't be disappointed. Just give a try!
100% Satisfaction Guarantee or Money Back!
Generic Latisse Real Shots




Full Original Product Annotation
 For the use of an Ophthalmologist or a Hospital or a Laboratory only.
For the use of an Ophthalmologist or a Hospital or a Laboratory only.
Bimatoprost Ophthalmic Solution /Careprost®
Composition:
Each ml contains:
Bimatoprost 0.3 mg
Benzalkonium Chloride Solution IP 0.01 % w/v
(As Preservative)
Aqueous Buffered Vehicleq.s.
Clinical Pharmacology:
Bimatoprost is a prostamide, a synthetic structural analog of prostaglandin F2-alpha (PGF2-alpha) with ocular hypotensive activity.
Mechanism of Action
Bimatoprost selectively mimics the effects of naturally occurring substances, prostamides. Bimatoprost is believed to lower intraocular pressure (I0P) in humans by increasing outflow of aqueous humor through both the trabecular meshwork and uveoscleral routes. Elevated IOP presents a major risk factor for glaucomatous field loss. The higher the level of IOP the greater the likelihood of optic nerve damage and visual field loss.
Pharmacokinetics
Bimatoprost penetrates the human cornea and sclera well in vitro. After once daily ocular administration of one drop of 0.03% bimatoprost to both eyes for two weeks, blood concentrations peaked within 10 minutes after dosing and declined to below the lower limit of detection (0.025 ng/ml) within 1.5 hours after dosing. Mean Cmax and AUC0-24hr values were similar on days 7 and 14 at approximately 0.08 ng/mL and 0.09 ng*hr/mL, respectively, indicating that steady state was reached during the first week of ocular dosing. After ocular administration, the systemic exposure of bimatoprost is very low with no accumulation overtime.
Bimatoprost is moderately distributed into body tissues and the systemic volume of distribution in humans at steady state was 0.67 l/kg. In human blood, bimatoprost resides mainly in the plasma. The plasma protein binding of bimatoprost is approximately 88%. Approximately 12% of bimatoprost remains unbound in human plasma.
Bimatoprost is the major circulating species in the blood once it reaches the systemic circulation following ocular dosing. Bimatoprost then undergoes oxidation, N- deethylation and glucuronidation to form a diverse variety of metabolites.
Following an intravenous dose of radiolabeled bimatoprost (3.12 mcg/kg) to healthy subjects, the maximum blood concentration of unchanged drug was 12.2 ng/mL and decreased rapidly with an elimination half life of approximately 45 minutes. The total blood clearance of bimatoprost was 1.5 L/hr/kg. Bimatoprost is eliminated primarily by renal excretion; up to 67% of an intravenous dose was excreted in the urine while 25% of the dose was excreted via the feces.
Indications:
Careprost is indicated for the reduction of elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension.
Contra-indications:
Hypersensitivity to bimatoprost or any other ingredient of the formulation.
Warnings and Precautions:
FOR EXTERNAL USE ONLY. NOT FOR INJECTION.
Bimatoprost ophthalmic solution has been reported to cause changes to pigmented tissues. These reports include increased pigmentation and growth of eyelashes and increased pigmentation of the iris and periorbital tissue (eyelid). These changes may be permanent.
Bimatoprost may gradually change eye color, increasing the amount of brown pigment in the iris by increasing the number of melanosomes (pigment granules) in melanocytes. The long term effects on the melanocytes and the consequences of potential injury to the melanocytes and/or deposition of pigment granules to other areas of the eye are currently unknown. The change in iris color occurs slowly and may not be noticeable for several months to years. Patients should be informed of the possibility of iris color change.
Bimatoprost ophthalmic solution contains the preservative benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation and may be reinserted 15 minutes following administration.
Benzalkonium chloride, which is commonly used as a preservative in ophthalmic products, has been reported to cause punctate keratopathy and/or toxic ulcerative keratopathy. Since bimatoprost ophthalmic solution contains benzalkonium chloride, monitoring is required with frequent or prolonged use in dry eye patients or where the cornea is compromised.
Eyelid skin darkening has also been reported in association with the use of bimatoprost. Bimatoprost may gradually change eyelashes; these changes include increased length, thickness, pigmentation, and number of lashes.
Patients who are expected to receive treatment in only one eye should be informed about the potential for increased brown pigmentation of the iris, periorbital tissue, and eyelashes in the treated eye and thus, heterochromia between the eyes. They should also be advised of the potential for a disparity between the eyes in length, thickness, and/or number of eyelashes.
There have been reports of bacterial keratitis associated with the use of multiple dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
Patients may slowly develop increased brown pigmentation of the iris. This change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil is expected to spread concentrically towards the periphery in affected eyes, but the entire iris or parts of it may also become more brownish. Until more information about increased brown pigmentation is available, patients should be examined regularly and, depending on the clinical situation, treatment may be stopped if increased pigmentation ensues. The increase in brown iris pigment is not expected to progress further upon discontinuation of treatment, but the resultant color change may be permanent. Neither nevi nor freckles of the iris are expected to be affected by treatment.
Bimatoprost should be used with caution in patients with active intraocular inflammation (e.g., uveitis). Macular edema, including cystoid macular edema, has been reported during treatment with bimatoprost ophthalmic solution. Bimatoprost should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Bimatoprost has not been studied in patients with compromised respiratory function and should therefore be used with caution in such patients. In clinical studies, in those patients with a history of a compromised respiratory function, no significant untoward respiratory effects have been seen. Bimatoprost has not been studied in patients with heart block more severe than first degree or uncontrolled congestive heart failure.
Bimatoprost has not been studied in patients with inflammatory ocular conditions, neovascular, inflammatory, angle-closure glaucoma, congenital glaucoma or narrowangle glaucoma.
If irritation occurs, the use of this formulation should be discontinued.
Bimatoprost / Careprost is not expected to affect the ability to drive and use machines. As with any ocular treatment, if transient blurred vision occurs at instillation, the patient should wait until the vision clears before driving or using machinery.
Pregnancy & Lactation
There are no adequate and well controlled studies of bimatoprost administration in pregnant women.
Because animal reproductive studies are not always predictive of human response, bimatoprost should be administered during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether bimatoprost is excreted in human milk, although in animal studies, bimatoprost has been shown to be excreted in breast milk. Because many drugs are excreted in human milk, caution should be exercised when bimatoprost is administered to a nursing woman.
Drug Interactions:
No interactions are anticipated in humans, since systemic concentrations of bimatoprost are extremely low (less than 0.2 ng/ml) following ocular dosing. Bimatoprost is biotransformed by any of multiple enzymes and pathways, and no effects on hepatic drug metabolising enzymes were observed in preclinical studies. Therefore, specific interaction studies with other medicinal products have not been performed with bimatoprost.
In clinical studies, bimatoprost was used concomitantly with a number of different ophthalmic beta-blocking agents without evidence of interactions. Concomitant use of bimatoprost and antiglaucomatous agents other than topical beta-blockers has not been evaluated during adjunctive glaucoma therapy.
Side effects:
The most commonly reported adverse effects with bimatoprost ophthalmic solution were conjunctival hyperemia, growth of eyelashes, ocular pruritus, ocular dryness, visual disturbance, worsening of visual acuity, ocular burning, foreign body sensation, eye pain, pigmentation of the periocular skin, blepharitis, cataract, superficial punctate keratitis, eyelid erythema, eyelid pruritus, ocular irritation, eyelash darkening, eye discharge, tearing, photophobia, allergic conjunctivitis, asthenopia, increases in iris pigmentation, conjunctival edema, corneal erosion, iritis, cystoid macular edema, eyelid retraction, retinal hemorrhage, and uveitis.
Systemic adverse events reported with Careprost were infections (primarily colds and upper respiratory tract infections), headache, abnormal liver function tests, hypertension, dizziness, peripheral edema, asthenia, and hirsutism.
Overdosage:
No information is available on overdosage in humans. If overdose with bimatoprost ophthalmic solution occurs, treatment should be symptomatic and supportive. In oral (by gavage) mouse and rat studies, doses up to 100 mg/kg/day did not produce any toxicity. This dose expressed as mg/m2 is at least 70 times higher than the accidental dose of one bottle of bimatoprost for a 10 kg child.
Dosage and Administration:
The recommended dosage is one drop in the affected eye(s) once daily in the evening. The dosage of bimatoprost ophthalmic solution should not exceed once daily since it has been shown that more frequent administration may decrease the intraocular pressure lowering effect.
Reduction of the intraocular pressure starts approximately 4 hours after the first administration with maximum effect reached within approximately 8 to 12 hours.

Bimatoprost may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic drug is being used, the drugs should be administered at least 5 minutes apart.
The dropper tip should not be allowed to touch any surface since this may contaminate the solution.
Pediatric use: Bimatoprost has only been studied in adults and therefore its use is not recommended in children or adolescents.
Hepatic and Renal impairment: Careprost has not been studied in patients with renal or moderate to severe hepatic impairment and should therefore be used with caution in such patients. In patients with a history of mild liver disease or abnormal ALT, AST and/or bilirubin at baseline, bimatoprost had no adverse effect on liver function over 24 months.
Storage:
Store at temperature not exceeding 25°C. Do not freeze. The solution should be used within one month after opening the container.
Presentation:
Careprost is available as 3 ml in plastic bottles.
Acme Plaza, Andheri-Kurla Road, Andheri (E), Mumbai 400 059, INDIA.