No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
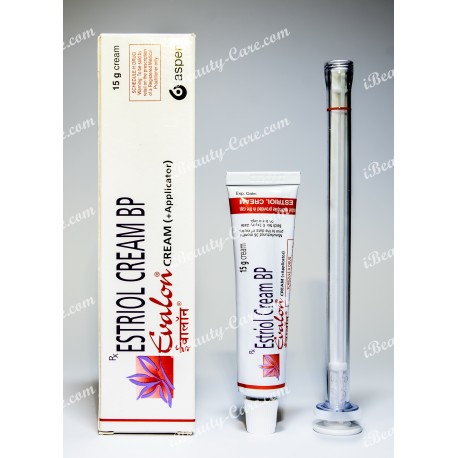
Generic Estrace vaginal cream
New
- Estrace is an estradiol vaginal cream which is a form of estrogen replacement therapy and is used to relieve the symptoms associated with vaginal menopause. Estradiol vaginal creams contain a form of synthetic estrogen hormone. The estrogen deficiency usually occurs after menopause but can occur to a younger woman with a hypo-functional ovary or whose ovary has been removed.
295 Items
More info

|
Basic Information
|
 |
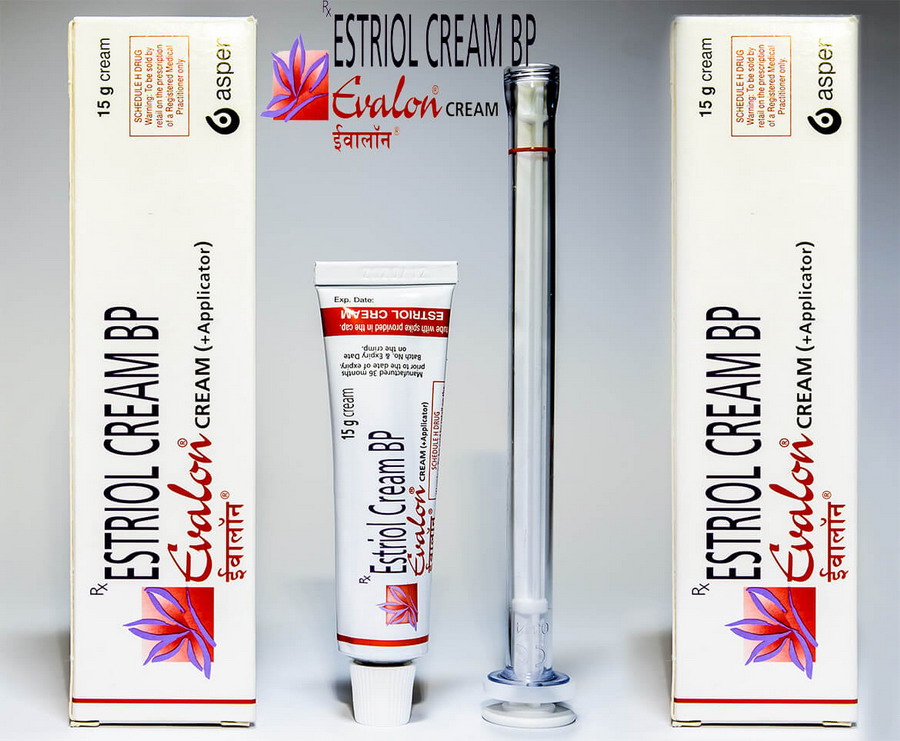
Generic Estrace vaginal cream (Evalon Cream) your smart decision during menopause.
Are you suffering from vaginal dryness, discomfort, and pain due to menopause?
There is good news for you. Generic Estrace vaginal cream (Evalon Cream) is hormonal vaginal creams that are clinically tested and approved for the treatment of vulvar and vaginal atrophy during menopause.
It is used intravaginally and helps normalize the epithelium of vaginal linings, restores microflora, and physiological pH in the vagina. As a result, it prevents and occurrence and development of infections, reduce inflammation,
dryness, pain, discomfort, and itching. Urination and urinary incontinence are also stabilized.
Just try Evalon (Estriol Cream) during menopause.
Because of the insufficient products of estrogen during menopause vaginal lining thins become dry as there is less blood flow to the tissue. This results in a disbalance of microflora and changes in PH. This is its turn disturbs the natural protective mechanisms causing more harmful bacteria to grow. Generic Estrace (Estriol cream) contains natural hormone estriol and restores hormone level and vaginal hydration. Estriol is a short-acting hormone that is useful in a local application for vaginal atrophy. 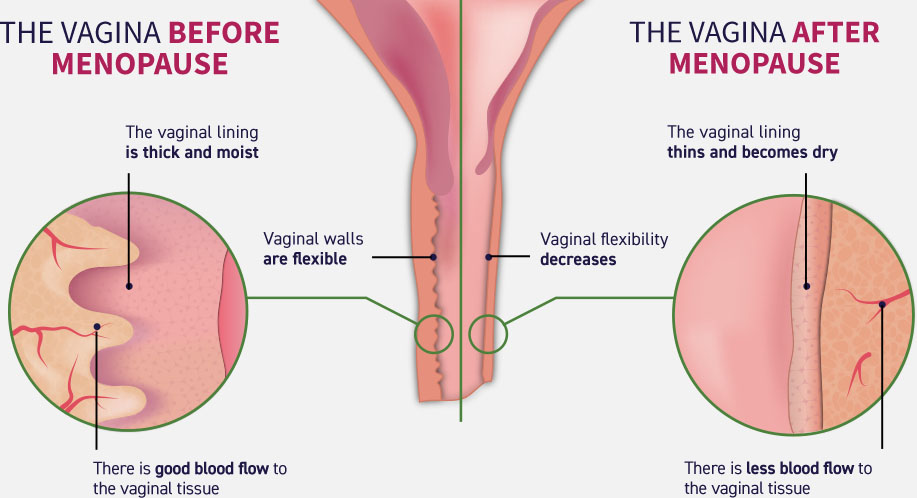
|
Why do you need to use Estrio cream? |
||||
|
 |
|||
|
How to Use Estriol vaginal cream |
||||
| Apply Estriol cream intravaginally using the applicator provided. | It is recommended to use Estriol cream once daily before bedtime. | One application contains 0.5mg of Evalon cream equivalent to 0.5mg Estriol. | One pack of 15g is suitable for 30 applications. Evalon cream is intended for the short-term treatment. | After the first week, your doctor may recommend to use cream once or twice weekly depending on the result of the first week of treatment. |
Generic Estrace weekly progress
Evalon Cream (generic Estrace vaginal cream) is intended for the short-term treatment. Do not use more than 1 pack without consultation with your physician.
After the second week, your doctor may decrease to decrease dose in a half to once or twice application weekly.
Full satisfaction is guaranteed!!!
Estrace vaginal cream and Evalon cream are identical products made by two different manufacturers. They contain the same active ingredient Estriol and are used for the same purposes. Both products provide great results in relieving the symptoms of vaginal atrophy.
- Estrace=Evalon
- Same active ingredients
- Same additives
- Same volume
- Same indications
- Same results for less money

Generic Estrace Estriol cream brief description
Generic Estrace vaginal cream (Estriol cream BP)
Qualitative and quantitative composition - The generic form of Estrace is Estradiol. 1g of the Estrace cream contains 1mg of Estradiol.
Action mechanism - Estradiol contains synthetic estrogen hormone. The hormone is naturally produced in the human body. In women, the estrogen level is higher and is produced primarily in the ovaries. Men have much lower estradiol levels in the body, and it is produced in the adrenal cortex and in the testes. Estrogens are not only very important for the development of female sexual organs (such as ovaries, uterus, vagina, and breasts), but also for their function. The approximately 28-day menstrual cycle is largely dependent on the changing hormone levels in the woman's blood. When the female body produces less estrogen, it suffers from several side effects including vaginal dryness, hardening of the vaginal tissues, and pain during intercourse. Estradiol cream contains synthetic estrogen hormone to supply it to the local tissue through topical application. The menopause symptoms include vaginal dryness, hardening of the vaginal tissues (also known as vaginal atrophy), and pain during intercourse. These creams are not intended to relieve other types of menopausal symptoms such as hot flashes, night sweats, and mood swings.
Indication - Estradiol is used primarily as a hormone replacement therapy in women with complaints of a deficiency of certain female sex hormones (the estrogens). The cream is used only to ease the effect of estrogen deficiency in the vaginal region (such as vaginal tissue hardening, vaginal dryness, and pain during sexual intercourse).
Mode of administration – This cream is used by inserting a measured amount of the cream into the vagina before bedtime. For the treatment of menopausal symptoms, daily 2.5 g of estrogen are sufficient. Higher doses are required for other indications such as breast tenderness. It is not advisable to have higher doses of Estradiol without consulting a physician.
Contraindication - Do not use the Estradiol cream if you have estrogen-related cancer. The cream is also contraindicated with asthma, epilepsy, hypertension, liver disease, thrombosis, and untreated bleeding. You should not use estradiol cream during pregnancy and lactation. Estradiol is not intended for the treatment of children.
Special cautions and warnings - You should never use estrogen creams without the advice and care of the doctor. When women use estrogen creams their blood levels of hormones need to be monitored regularly. Doctors must evaluate your medical history and current medications before prescribing the estrogen creams.
Overdose symptoms of estrogen cream include vomiting, vaginal bleeding, lethargy, dizziness, and abdominal pain. If you develop these symptoms after using an estrogen cream you should contact their doctor immediately.
Generic Estrace creams are not recommended for women who have a history of estrogen-dependent cancers, such as breast cancer. This is because the use of estrogen replacement therapy may increase the chances of recurring cancer.
Interaction - The Estradiol used as a cream for external use only has less interaction with other drugs compared to the ingested dose. Estradiol cream is effective for menopause-related complaints. The degradation of estradiol after external application can be affected by the simultaneous use of drugs that activate the degrading enzyme system. In these cases, Estradiol gets degraded quickly. Such drugs include anti-epileptic medicines such as carbamazepine, phenobarbital, and phenytoin; the tuberculosis drugs rifabutin and rifampicin; and the antiviral drugs efavirenz, nelfinavir, nevirapine, and ritonavir. Several antidepressants also interact with Estradiol. Drug interaction can also lead to a reduced effect of estradiol. Some of these interactions may cause menstrual bleeding.
Side effects - Generic Estracecreams can cause side effects that include mild vaginal bleeding, breast pain, headaches and bloating. Long-term use of estrogen creams can also cause thickening of the uterine lining, which can increase the risk of endometrial cancer. Estrogen cream may interact with some types of prescription medications, especially anticoagulants, cyclosporine, and barbiturates.
Interesting facts
-
Doctors recommend that women try over-the-counter lubrication creams (which do not contain estrogen) to relieve the symptoms of vaginal menopause before resorting to estrogen creams.
-
Estrogen creams are not recommended for women who are currently on other forms of estrogen replacement therapy (pills, patches or vaginal rings).
-
Women who have not undergone a hysterectomy are often prescribed progestin cream in addition to estrogen cream to reduce the increased risks of endometrial cancer that are associated with the use of estrogen.
-
Over-the-counter estrogen creams have not been evaluated by the FDA and they may have unknown composition. The dose of estrogen in the over-the-counter creams can vary widely and are not always consistent. You may get an overdose or lower dose of the medication with these creams.
Generic Estrace Real Shots

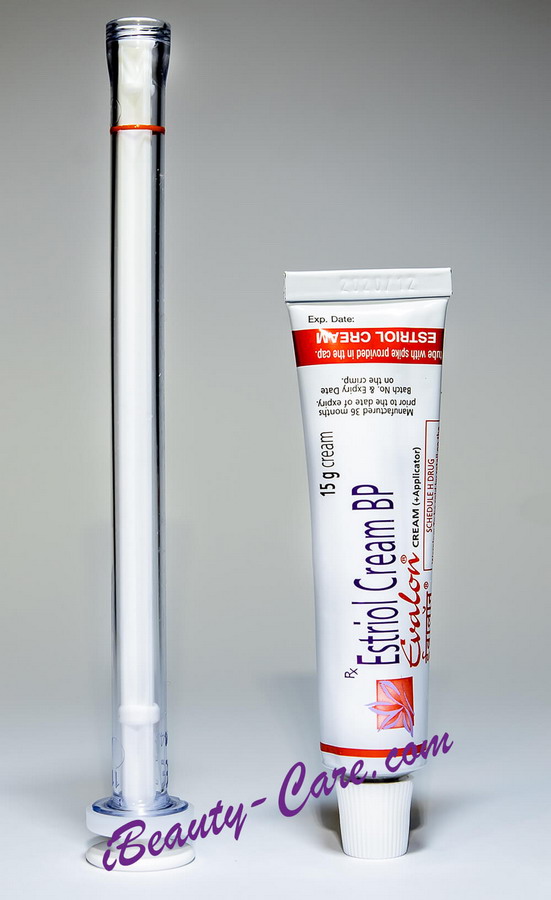

Full Original Product Annotation
 FOR INTRAVAGINAL USE ONLY
FOR INTRAVAGINAL USE ONLY
NAME OF THE MEDICINAL PRODUCT
Estriol 1mg cream / Generic Estrace / Evalon Cream
COMPOSITION
Each gram of Estriol cream (Generic Estrace) contains:
Estriol BP 1mg
Chlorhexidine Hydrochloride BP (as preservative) 0.1 mg in a cream base q.s.
DOSAGE FORM
Cream.
Homogeneous, smooth, white to nearly white mass of creamy consistency.
INDICATIONS
Estrogen deficiency symptoms due to menopause.
DOSE. METHOD OF ADMINISTRATION AND USAGE
Evalon Cream (Generic Estrace) is an estrogen-only product that may be given to women with or without a uterus.
Posology
For atrophy of the lower urogenital tract:
1 application per day for the first weeks, followed by a gradual reduction, based on relief of symptoms, until a maintenance dosage (e.g. 1 application twice a week) is reached.
Method of administration
Evalon Cream should be administered intra vaginally by means of a calibrated applicator before retiring at night.
1 application (applicator filled to the ring mark) contains 0.5 g Evalon Cream which corresponds to
0.5 mg estriol.
Instructions for use for the patient
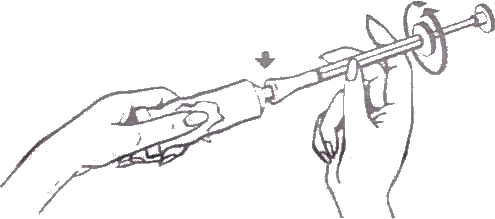
- Remove cap from the tube, invert it, and use the sharp point to open the tube.
- Screw the end of the applicator onto the tube. Make sure the plunger is fully inserted into the barrel.
- Squeeze tube slowly to fill the applicator with the cream until the plunger stops (at the red ring, see arrows in the picture below).
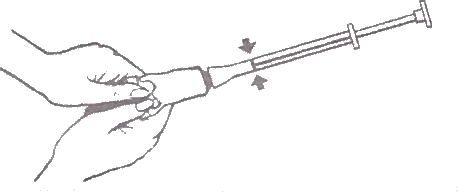
- Unscrew applicator from tube and put cap back on tube.
- To apply cream, lie down, insert end of applicator deep into the vagina.
- Slowly push plunger all the way in until applicator is empty.
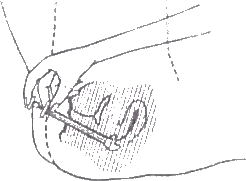
- After use. pull plunger out of barrel beyond the point of resistance and wash both in warm, soapy water. Do not use detergents. Rinse well afterwards. DO NOT PUT THE APPLICATOR IN HOT OR BOILING WATER.
- The applicator can be re-assembled by fully inserting the plunger into the barrel beyond the point where resistance is felt. Discard the applicator once the tube is empty.
CONTRA-INDICATIONS
- Known, past or suspected breast cancer
- Known or suspected estrogen-dependent malignant tumors (e.g. endometrial cancer)
- Undiagnosed genital bleeding
- Untreated endometrial hyperplasia
- Previous or current venous thromboembolism (deep venous thrombosis, pulmonary embolism)
Known thrombophilic disorders (e.g. protein C, protein S, or antithrombin deficiency, see WARNINGS AND PRECAUTIONS)
- Active or recent arterial thromboembolic disease (e.g. angina, myocardial infarction)
- Acute liver disease, or a history of liver disease as long as liver function tests failed to return to normal
- Hypersensitivity to the active substance or to any of the excipients listed in section LIST OF EXCIPIENTS.
- Porphyria
WARNINGS AND PRECAUTIONS
For the treatment of postmenopausal symptoms, HRT should only be initiated for symptoms that adversely affect quality of life. In all cases, a careful appraisal of the risks and benefits should be undertaken at least annually and HRT should only be continued as long as the benefit outweighs the risk.
Evidence regarding the risks associated with HRT in the treatment of premature menopause is limited. Due to the low level of absolute risk in younger women, however, the balance of benefits and risks for these women may be more favorable than in older women.
Medical examination/follow-up
Before initiation or reinstituting HRT, a complete personal and family medical history should be taken. Physical (including pelvic and breast) examination should be guided by this and by the contraindications and warnings for use. During treatment, periodic check-ups are recommended of a frequency and nature adapted to the individual woman.
Women should be advised what changes in their breast should be reported to their doctor or nurse (see ‘Breast cancer’ below). Investigations, including appropriate imaging tools, e.g. mammography, should be carried out in accordance with currently accepted screening practices, modified to the clinical needs of the individual.
Conditions which need supervision
If any of the following conditions are present, have occurred previously, and/or have been aggravated during pregnancy or previous hormone treatment, the patient should be closely supervised. It should be taken into account that these conditions may recur or be aggravated during treatment with Evalon Cream, in particular:
- Leiomyoma (uterine fibroids) or endometriosis
- Risk factors for thromboembolic disorders (see below)
- Risk factors for estrogen dependent tumors, e.g. 1st degree heredity for breast cancer
- Hypertension
- Liver disorders (e.g. liver adenoma)
- Diabetes mellitus with or without vascular involvement
- Cholelithiasis
- Migraine or (severe) headache
- Systemic lupus erythematosus
- A history of endometrial hyperplasia (see below)
- Epilepsy
- Asthma
- Otosclerosis
Reasons for immediate withdrawal of therapy:
Therapy should be discontinued in case a contraindication is discovered and in the following situations:
- Jaundice or deterioration in liver function
- Significant increase in blood pressure
- New onset of migraine-type headache
- Pregnancy
In order to prevent endometrial stimulation, the daily dose should not exceed 1 application (0.5 mg estriol) nor should this maximum dose be used for longer than several weeks. One epidemiological study has shown that long-term treatment with low doses of oral estriol, but not vaginal estriol, may increase the risk for endometrial cancer. This risk increased with the duration of treatment and disappeared within one year after the treatment was terminated. The increased risk mainly concerned less invasive and highly differentiated tumors. Vaginal bleeding during medication should always be investigated. The patient should be informed to contact a doctor if vaginal bleeding occurs.
Breast cancer
The overall evidence suggests an increased risk of breast cancer in women taking combined estrogen- progestagen and possibly also estrogen-only HRT, that is dependent on the duration of taking HRT. Combined estrogen-progestagen therapy
The randomized placebo-controlled trial (Women’s Health Initiative study (WHI)), and epidemiological studies are consistent in finding an increased risk of breast cancer in women taking combined estrogen-progestagen for HRT that becomes apparent after about 3 years (see UNDESIRABLE EFFECTS) Estrogen-only therapy
The WHI trial found no increase in the risk of breast cancer in hysterectomized women using estrogen- only HRT. Observational studies have mostly reported a small increase in risk of having breast cancer diagnosed that is substantially lower than that found in users of estrogen-progestagen combinations (see
UNDESIRABLE EFFECTS
The excess risk becomes apparent within a few years of use but returns to baseline within a few (at most five) years after stopping treatment. HRT, especially estrogen-progestagen combined treatment, increases the density of mammographic images which may adversely affect the radiological detection of breast cancer. Clinical studies reported that the likelihood of developing increased mammographic density was lower in subjects treated with estriol than in subjects treated with other estrogens. It is unknown whether Evalon Cream carries the same risk. In a population-based casecontrol study in 3,345 women with invasive breast cancer and 3,454 controls, estriol was found not to be associated with an increased risk of breast cancer, in contrast to other estrogens. However, the clinical implications of these findings are as yet unknown. Therefore, it is important that the risk of being diagnosed with breast cancer is discussed with the patient and weighed against the known benefits of HRT.
Ovarian cancer
Ovarian cancer is much rarer than breast cancer. Long-term (at least 5-10 years) use of estrogen-only HRT products has been associated with a slightly increased risk of ovarian cancer (see UNDESIRABLE EFFECTS). Some studies including the WHI trial suggest that the long-term use of combined HRTs may confer a similar, or slightly smaller, risk (see UNDESIRABLE EFFECTS). It is uncertain whether long-term use of low potency estrogens (such as Evalon Cream) confers a different risk than other estrogen- only products.
Venous thromboembolism
HRT is associated with a 1.3-3-fold risk of developing venous thromboembolism (VTE), i.e. deep vein thrombosis or pulmonary embolism. The occurrence of such an event is more likely in the first year of HRT than later (see UNDESIRABLE EFFECTS). These studies did not include Evalon Cream and, in the absence of data, it is unknown whether Evalon Cream carries the same risk.
Patients with known thrombophilic states have an increased risk of VTE and HRT may add to this risk. HRT is therefore contraindicated in these patients (see CONTRA-INDICATIONS).
Generally recognized risk factors for VTE include, use of estrogens, older age, major surgery, prolonged immobilization, obesity (Body Mass Index >30 kg/ma), pregnancy/postpartum period, systemic lupus erythematosus (SLE), and cancer. There is no consensus about the role of varicose veins in VTE.
As in all postoperative patients, prophylactic measures need be considered to prevent VTE following surgery. If prolonged immobilization is to follow elective surgery temporarily stopping HRT 4 to 6 weeks earlier is recommended.
Treatment should not be restarted until the woman is completely mobilized.
In women with no personal history of VTE but with a first degree relative with a history of thrombosis at young age, screening may be offered after careful counseling regarding its limitations (only a proportion of thrombophilic defects are identified by screening). If a thrombophilic defect is identified which segregates with thrombosis in family members or if the defect is ‘severe’ (e.g., antithrombin, protein S, or protein C deficiencies or a combination of defects) HRT is contraindicated. Women already on anticoagulant treatment require careful consideration of the benefit-risk of use of HRT.
If VTE develops after initiating therapy, the drug should be discontinued. Patients should be told to contact their doctors immediately when they are aware of a potential thromboembolic symptom (e.g. painful swelling of a leg, sudden pain in the chest, dyspnea).
Coronary artery disease (CAD)
There is no evidence from randomized controlled trials of protection against myocardial infarction in women with or without existing CAD who received combined estrogen-progestagen or estrogen-only HRT. Combined estrogen-progestagen therapy
The relative risk of CAD during use of combined estrogen-progestagen HRT is slightly increased. As the baseline absolute risk of CAD is strongly dependent on age, the number of extra cases of CAD due to estrogen-progestagen use is very low in healthy women close to menopause, but will rise with more advanced age.
Estrogen-only
Randomized controlled data found no increased risk of CAD in hysterectomized women using estrogen-only therapy.
Ischemic stroke
Combined estrogen-progestagen and estrogen-only therapy are associated with an up to 1,5-fold increase in risk of ischemic stroke. The relative risk does not change with age or time since menopause. However, as the baseline risk of stroke is strongly age-dependent, the overall risk of stroke in women who use HRT will increase with age (see UNDESIRABLE EFFECTS).
Otherconditions
- Estrogens may cause fluid retention, and therefore patients with cardiac or renal dysfunction should be carefully observed.
- Estriol is a weak gonadotropin inhibitor without other significant effects on the endocrine system.
- HRT use does not improve cognitive function. There is some evidence of increased risk of probable dementia in women who start using continuous combined or estrogen-only HRT after the age of 65.
- Evalon Cream / Generic Estrace is not intended for contraceptive use.
- Evalon Cream / Generic Estrace contains acetyl alcohol and stearyl alcohol. This may cause local skin reactions (e.g. contact dermatitis).
DRUG INTERACTIONS
No examples of interactions between Evalon Cream and other medicines have been reported in clinical practice. Although data are limited, interactions between Generic Estrace and other medicinal products may occur. The following interactions have been described with use of combined oral contraceptives which may also be relevant for Evalon Cream.
The metabolism of estrogens may be increased by concomitant use of substances known to induce drug-metabolizing enzymes, specifically cytochrome P450 enzymes, such as anticonvulsants (e.g. phenobarbital, phenytoin, carbamazepin) and anti-infectives (e.g. rifampicin, rifabutin, nevirapine, efavirenz). Ritonavir and nelfinavir, although known as strong inhibitors, by contrast exhibit inducing properties when used concomitantly with steroid hormones.
Herbal preparations containing St John’s wort (Hypericum Perforatum) may induce the metabolism of estrogens. Clinically, an increased metabolism of estrogens may lead to decreased effect and changes in the uterine bleeding profile.
PREGNANCY AND LACTATION
Fertility
Evalon Cream is intended for the treatment in postmenopausal (naturally and surgically induced) women only. Evalon Cream is not indicated during pregnancy. If pregnancy occurs during medication with Evalon Cream, treatment should be withdrawn immediately. The results of most epidemiological studies to date relevant to inadvertent fetal exposure to estrogens indicate no teratogenic or fetotoxic effects.
Lactation
Generic Estrace is not indicated during lactation. Estriol is excreted in breast milk and may decrease milk production.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
There is no information to suggest that Evalon Cream affects a patient’s ability to drive or operate machinery.
UNDESIRABLE EFFETCS
From literature and safety surveillance monitoring, the following adverse reactions have been reported:
|
System organ class |
Adverse reactions* |
|
Metabolism and nutrition disorders |
Fluid retention |
|
Gastrointestinal disorders |
Nausea |
|
Reproductive system and breast disorders |
Breast discomfort and pain Postmenopausal spotting Cervical discharge |
|
General disorders and administration site conditions |
Application site irritation and pruritus Flu-like symptoms |
*MedDRA version 15.1
These adverse reactions are usually transient, but may also be indicative of too high a dosage.
Other adverse reactions have been reported in association with estrogen/progestagen treatment:
- Estrogen-dependent neoplasms benign and malignant, e.g. endometrial cancer. For further information, see sections "CONTRAINDICATIONS" and "WARNINGS AND PRECAUTIONS'
- Gall bladder disease
- Skin and subcutaneous disorders: chloasma, erythema multiform, erythema nodosum, vascular purpura
- Probable dementia over the age of 65 (see WARNINGS AND PRECAUTIONS) Breast cancer risk
An up to 2-fold increased risk of having breast cancer diagnosed is reported in women taking combined estrogen-progestagen therapy for more than 5 years. Any increased risk in users of estrogen-only therapy is substantially lower than that seen in users of estrogen-progestagen combinations. The level of risk is dependent on the duration of use (see WARNINGS AND PRECAUTIONS). Results of the largest randomized placebo-controlled trial (WHI-study) and largest epidemiological study (MWS) are presented.
Million Women study- Estimated additional risk of breast cancer after 5 years’ use
|
Age range (years) |
Additional cases per 1000 never users of HRT over a 5 year period* |
Risk ratio# |
Additional cases per 1000 HRT users over 5 years (95%CI) |
|
Estrogen only HRT |
|||
|
50-65 |
9-12 |
1.2 |
1-2 (0-3) |
|
Combined estrogen-progestagen |
|||
|
50-65 |
9-12 |
1.7 |
6 (5-7) |
#Overall risk ratio. The risk ratio is not constant but will increase with increasing duration on use.
* Taken from baseline incidence rates in developed countries.
US WHI - additional risk of breast cancer after 5 years’ use
|
Age range (years) |
Incidence per 1000 women in placebo arm over 5 years |
Risk ratio & 95%CI |
Additional cases per 1000 HRT users over 5 years (95%CI) |
|
CEE estrogen-only |
|||
|
50-79 |
21 |
0.8 (0.7-1.0) |
-4 (-6 - 0)* |
|
CEE+MPA estrogen & progestagen* |
|||
|
50-79 |
17 |
1.2 (1.0-1.5) |
+4 (0 - 9) |
*When the analysis was restricted to women who had not used HRT prior to the study there was no increased risk apparent during the first 5 years of treatment: after 5 years the risk was higher than in non-users.
* WHI study in women with no uterus, which did not show an increase in risk of breast cancer.
Ovarian cancer
Long-term use of estrogen-only and combined estrogen-progestagen HRT has been associated with a slightly increased risk of ovarian cancer. In the Million Women Study 5 years of HRT resulted in 1 extra case per 2500 users.
Risk of venous thromboembolism
HRT is associated with a 1.3-3-fold increased relative risk of developing venous thromboembolism (VTE), i.e. deep vein thrombosis or pulmonary embolism. The occurrence of such an event is more likely in the first year of using HRT (see WARNINGS AND PRECAUTIONS). Results of the WHI studies are presented:
WHI Studies - Additional rick of VTE over 5 years’ use
|
Age range (years) |
Incidence per 1000 women in placebo arm over 5 years |
Risk ratio and 95%CI |
Additional cases per 1000 HRT users |
|
Oral estrogen-only* |
|||
|
50-59 |
7 |
1.2 (0.6-2.4) |
1 (-3-10) |
|
Oral combined estrogen-progestagen |
|||
|
50-59 |
4 |
2.3 (1.2-4.3) |
5 (1 -13) |
* Study in women with no uterus
Risk of coronary artery disease
The risk of coronary artery disease is slightly increased in users of combined estrogen-progestagen HRT over the age of 60 (see WARNINGS AND PRECAUTIONS).
Risk of ischemic stroke
The use of estrogen-only and estrogen-progestagen therapy is associated with an up to 1.5 fold increased relative risk of ischemic stroke. The risk of hemorrhagic stroke is not increased during use of HRT. This relative risk is not dependent on age or on duration of use, but as the baseline risk is strongly age dependent, the overall risk of stroke in women who use HRT will increase with age, see WARNINGS AND PRECAUTIONS.
WHI studies combined – Additional risk of ischemic stroke* over 5 years’ use
|
Age range (years) |
Incidence per 1000 women in placebo arm over 5 years |
Risk ratio and 95%CI |
Additional cases per 1000 HRT users over 5 years |
|
50-59 |
8 |
1.3 (1.1-1.6) |
3(1-5) |
*no differentiation was made between ischemic and hemorrhagic stroke.
OVERDOSE
The acute toxicity of estriol in animals is very low. Overdose with Evalon Cream after vaginal administration is unlikely. However, in cases where large quantities are ingested, nausea, vomiting, and withdrawal bleeding in females may occur. No specific antidote is known. Symptomatic treatment can be given if necessary.
PHARMACODYNAMIC AND PHARMACOKINETIC PROPERTIES
Pharmacodynamic properties
Pharmacotherapeutic group: natural and semisynthetic estrogens
АТС code: G03CA04
Mechanism of action
Evalon Cream / Generic Estrace contains the natural female hormone estriol. Unlike other estrogens, estriol is short acting since it has only a short retention time in the nuclei of endometrial cells. It substitutes for the loss of estrogen production in menopausal women and alleviates menopausal symptoms. Estriol is particularly effective in the treatment of urogenital symptoms. In case of atrophy of the lower urogenital tract estriol induces the normalization of the urogenital epithelium and helps to restore the normal microflora and the physiological pH in the vagina. As a result, it increases the resistance of the urogenital epithelial cells to infection and inflammation reducing vaginal complaints such as dyspareunia, dryness, itching, vaginal and urinary infections, miction complaints and mild urinary incontinence.
Clinicaltrialinformation
- Relief of menopausal symptoms was achieved during the first weeks of treatment.
- Vaginal bleeding after treatment with Evalon Cream has only rarely been reported.
Pharmacokinetic properties
Absorption
Intravaginal administration of estriol ensures optimal availability at the site of action. Estriol is also absorbed into the general circulation, as is shown by a sharp rise in the plasma levels of unconjugated estriol.
Distribution
Peak plasma levels are reached 1 -2 hours after application. After vaginal application of 0.5 mg estriol, Cmax is approximately 100 pg/ml, Cmin is approximately 25 pg/ml and Cavarage is approximately 70 pg/ml. After 3 weeks of daily administration of 0.5 mg vaginal estriol, Caverage has decreased to 40 pg/ml.
Biotransformation
Nearly all (90%) estriol is bound to albumin in the plasma and, in contrast with other estrogens, hardly any estriol is bound to sex hormone-binding globulin. The metabolism of estriol consists principally of conjugation and deconjugation during the enterohepatic circulation.
Elimination
Estriol, being a metabolic end product, is mainly excreted via the urine in the conjugated form. Only a small part (± 2%) is excreted via the feces, mainly as unconjugated estriol.
LIST OF EXCIPIENTS
- Octyldodecanol
- Cetyl palmitate
- Glycerol
- Cetyl alcohol
- Stearyl alcohol
- Polysorbate 60
- Sorbitan stearate
- Lactic acid
- Chlorhexidine dihydrochloride
- Sodium hydroxide
- Purified water
INCOMPTIBILITIES
Not Applicable
SHELF- LIFE
36 months
STORAGE
Store below 25°C, Do not Freeze.
Keep out of reach and sight of Children.
PACKAGING INFORMATION
Evalon Cream is filled in collapsible aluminium tubes of 15, 30 or 50 grams. Not all pack sizes may be marketed. The tubes are provided with a polyethylene screw cap.
The СЕ-marked applicator consists of a styrene acrylonitril barrel and a polyethylene plunger. Each tube is packed, together with an applicator in a cardboard box.
INSTRUCTIONS FOR USE/HANDLING
Any unused medicinal product, the applicator or waste material should be disposed of in accordance with local requirements.
Manufactured by:
Aspen Global Incorporated, Mauritius
Under license holding of Aspen Pharma Trading Limited, Ireland at
Aspen Bad Oldesloe GmbH
Industriestrasse 32 - 36, 23843 Bad Oldesloe Germany
Imported & Marketed in India by :
Sandoz Private Limited
Regd. Office : Sandoz House, Dr. A. B. Road,
Worli, Mumbai - 400 018
Under License No.: IL/FF-000101-FF-747
To report product complaint or Adverse Drug Reaction email us on sandoz-in.Pharmacovigilance@sandoz.com For further details contact:
Office : Sandoz House, Dr. A. B. Road, Worli, Mumbai - 400 018