No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
Retin-A cream 0.05% 20g
New
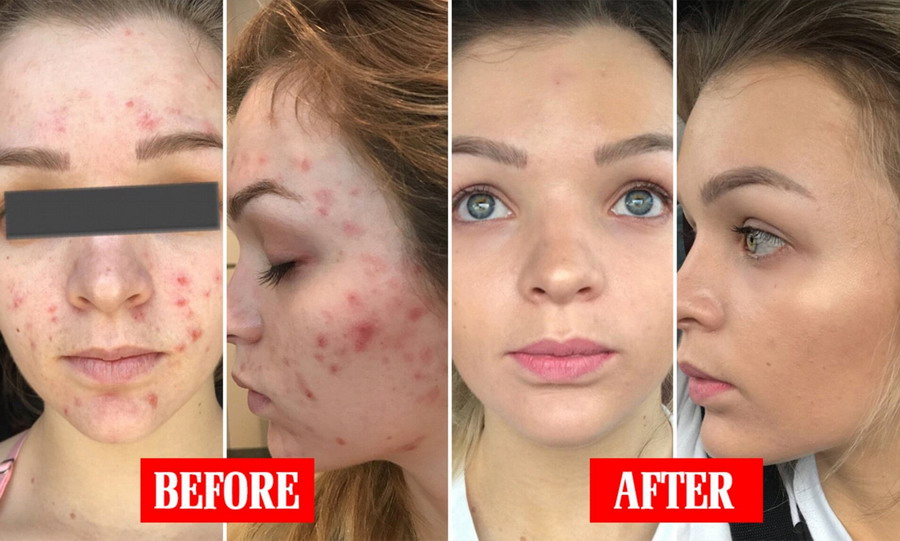
Retin-A cream contains the active ingredient Tretinoin, which is a form of vitamin A. This product is used primarily for the treatment of acne vulgaris. It helps the skin to renew itself by accelerating the process of skin renewal (production of new skin cells and replacing the old, aged, and dead cells).
290 Items
More info


|
Basic Information
|
 |
Are you still troubled by these problems?
Long and thick eyelashes are always a desire of all women. Bimatoprost is the only available synthetic compound approved to treat hypotrichosis, a condition characterized by insufficient growth of eyelashes, their shortness and thinness.
There many other conditions that may affect eyelashes like madarosis, blepharitis which require an approach by specialized medical staff. Bimatoprost works by extending the growth phase of lashes and increasing hair bulb thickness.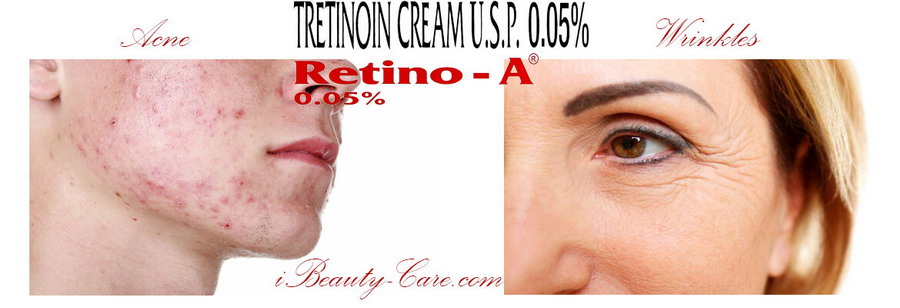
Activate Eyelashes Growth Power
If we compare the results before and after using Generic Latisse, we can be sure that Bimatoprost is amazing solutions that provide really great results in just 8 weeks. There are thousands of women all over the globe who can now enjoy beautiful, thick and dark eyelashes. An amazing result is guaranteed! All that you need is to use the solution on a daily basis and the desired result will not keep you waiting long. Latisse is an ideal solution for wispy and fine lashes.
|
Why do you need to use generic Latisse? |
||||
|
 |
|||
|
How to Use Generic Latisse |
||||
 |
 |
 |
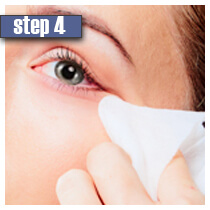 |
 |
| Wash and clean your face from makeup. Contact lenses should also be removed prior to using Latisse. For best results, apply the solution each day at night at the same time. | Place only one drop of solution on the application. Hold the applicator in a horizontal position. | After that draw the applicator tip carefully along the skin of the upper eyelid where the eyelashes meet the skin. Do not apply the solution to the lower lashes and eyelids. | Remove excess of solution using a tissue or a cotton swab. If you don't wipe off an excessive solution, side effects are likely to occur. | Dispose of the application. For the next day repeat the procedure with a new sterile application. In case you wear contact lenses, you may put them again in 15 minutes. Keep the applicators and tip of the bottle with solution away from contaminated area. |
Latisse Generic weekly progress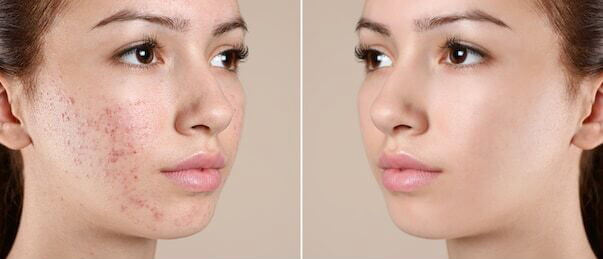
The results may vary depending on many factors such as age, eyelashes condition, and many others. According to the results of clinical studies, first visible results are achieved within 8 weeks.
- 1st week – 5% growth (Bimatoprost). Beginning of treatment. The results are still not visible.
- 4th week – 15% growth and some women start to notice first results. Their lashes become more longer.
- 8th week – 50% growth and women confirm their lashes are significantly fuller and darker.
- 12th week – 69% growth with Bimatoprost. The lashes continue to grow longer, fuller, and darker.
- 16th week – 78%-82% growth with Bimatoprost. Women see really great results and are fully satisfied with the results of treatment. Eyelash's fullness is more than doubled.
- 20th week – 82-106% growth with Bimatoprost. The growth is stabilized and maintained.
Full satisfaction is guaranteed!!!
Retin-A brief description
Product name - Retin-A 0.05% cream by Johnson and Johnson
Qualitative and quantitative composition – Retin-A cream contains 0.05% tretinoin as the main compound. Other additives are stearyl alcohol - 3%, sorbic acid - 0.2%, and butylhydroxytoluene - 0.1%.
Action mechanism - The precise mechanism of action of Retin-a cream (tretionin) is still not known. But different evidence suggests that Tretinoin works by partial improving the collagen deficiency of the skin. It has a complex mode of action. It also prevents the dermal matrix to get damaged by exposure to UV. Tretinoin fastens the death of the damaged and old cells present on the skin thereby making way to the rapid growth of new skin cells.
Indication - Retin-A cream 0.05% is indicated in the following conditions -
-
Acne
-
Wrinkles
-
Dry and rough spots on the skin
-
Small skin bumps
-
Cancer of white blood cells
Mode of administration - Retin-A cream should be applied regularly until you get the desired results. You must apply the gel with the clean and dry applicator on clean and dry skin. Apply the cream as a thin layer on the affected skin area and not in any other place. Do not try to add more than the necessary amount of this cream and non-affected areas should not come in contact with this cream. Do not wash the treated area for at least 1 hour after the application of this preparation. Avoid using other skin products on the treated area for at least 1 hour. Use the cream daily in the evening. Start using Retin-A cream or gel only after consulting your doctor.
Contraindication - Retin-A cream should not be indicted to people hypersensitive to it or any other component of the preparation. It also should not be given to pregnant women, breastfeeding women, children (up to 12 years), children up to 18 years (during treatment with sunlight), over the age of 50 years (during treatment with sunlight).
Special cautions and warnings - Retin-A cream containing cream should be used cautiously when a patient has alcoholism, chronic intoxication, chronic renal diseases, eczema, liver failure, pancreatic diabetes, pancreatitis, seborrheic dermatitis, and sunburn. It can increase sensitivity to cold, air-flow, and sunlight. Avoid prolonged exposure to sunlight, as well as UV-A reflector tubes. Do not wash your face excessively. Abrasive soap, cleansers, medicated gel or lotions can increase skin irritation. Retin-A cream should not be used during pregnancy or lactation without medical advice. Because of the possible adverse effects on the infant, the use of it during breastfeeding is not recommended.
Interaction - Tretinoin may affect the following medications: diuretics; Tetracycline group of antibiotics; other drugs used in acne, sunburn, and other skin diseases (such as Minocycline, Doxycycline, Demeclocycline); Antibiotics like ciprofloxacin, ofloxacin; Sulfates such as Bactrim, Septra, Cotrim and others; psychotic drugs like Chlorpromazine, Prochlorperazine, Fluphenazine, Promethazine, Perphenazine and others. However, the interaction between two medications does not always mean that you have to stop the use of one medication. Often the interaction may be minor and it is always advisable to consult with your doctor about how interactions can be managed.
Side effects - Retin-A has some common side effects, but mostly they are manageable and not too serious. It is normal for some skin reactions to occur. So make sure you know the difference between mild and severe side effects of taking Tretinoin containing skin ointments (creams or gels). If necessary, you may have to take the necessary action. Mild side effects include:
-
Burning sensation on the applied area
-
Dry skin
-
Redness of skin
-
Skin itching
-
Warm sensation
Severe Retin-A side effects are rare and include blistering and severe irritation. If you feel severe irritation, report it to your physician immediately. You may not be able to continue treatment with Tretinoin if you experience severe side effects. Sometimes darkening of the skin may happen as a temporary side effect of the drug when applied repeatedly.
Interesting facts
-
Avoid contact of the cream with the eyes. Rinse with cold water immediately if the cream accidentally comes in contact with your eyes.
-
Never double your dose. If you missed taking the medicine, take one dose as soon as you remember. But do not do it when it's almost time for your next dose. In that case, take your next dose the next day in the usual time.
-
In case of overdose with severe and persistent nausea, vomiting and diarrhea contact your doctor.
Retin-A Real Shots


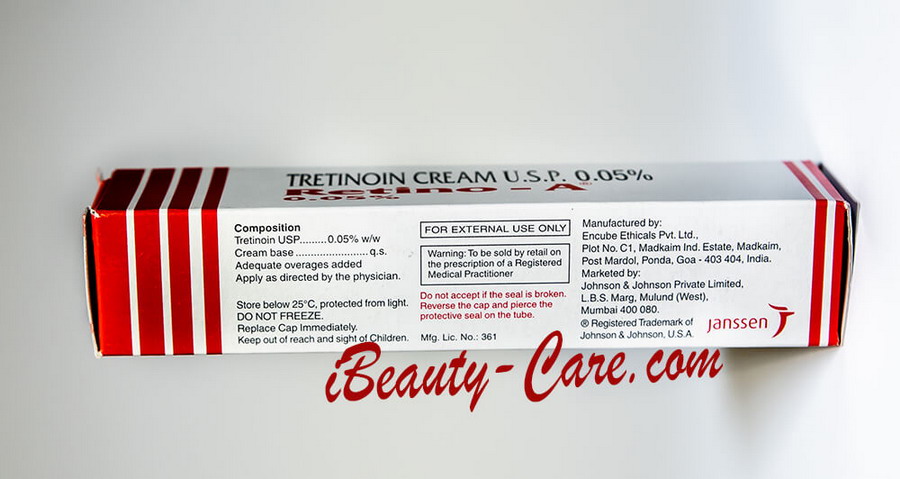
Full Original Product Annotation
RETINO-A cream / RETIN-A cream
 COMPOSITION
COMPOSITION
RETINO-A Cream contains tretinoin in the following strengths by weight: 0.025% & 0.05%
For excipients, see section List of excipients.
PHARMACEUTICAL FORM
RETIN-A is a white cream.
CLINICAL PARTICULARS
Therapeutic Indications
RETINO-A® is indicated as topical therapy for the treatment of acne vulgaris.
Posology and Method of Administration
Adults
RETINO-A® should be applied once daily in the evening before bedtime in only a sufficient quantity to lightly cover the entire affected areas. Prior to treatment with RETINO-A® areas being treated should be thoroughly cleansed with water and a mild, non-medicated soap. The treated area should be washed no more than twice a day. After washing, the skin should be dried gently and completely without rubbing it. Areas of the skin being treated should be allowed to dry for at least 20 to 30 minutes before application of RETINO-A®.
Application of RETINO-A® may cause a feeling of warmth and transitory stinging. When administered according to recommended guidelines, RETINO-A® may produce a slight erythema similar to that of mild sunburn. In cases where it is necessary to temporarily discontinue therapy or reduce the frequency of application, therapy should be resumed or the frequency of application increased when the patient becomes able to tolerate the treatment. Frequency of application should be closely monitored by careful observation of the clinical therapeutic response and skin tolerance.
Excess application of RETINO-A® does not provide more rapid or better results. In fact, marked redness, peeling or discomfort can occur. If excess application occurs accidentally or through over-enthusiastic use, RETINO-A® should be discontinued tor several days before resuming therapy.
Therapeutic effects may be noticed after two to three weeks of use but more than six weeks of therapy may be required before definite beneficial effects are seen. During the early weeks of treatment, an apparent exacerbation of inflammatory lesions may occur. This is due to the action of the medication on deep, previously unseen lesions and should not be considered a reason to discontinue therapy. Once a satisfactory response has been obtained, it may be possible to maintain this improvement with less frequent applications.
Patients treated with Retin-a cream may use cosmetics and moisturizers, but the areas of the skin to be treated should be cleansed thoroughly before application of RETINO-A® (see section on Special warnings and special precautions for use).
Children
Safety and effectiveness have not been established in children.
Contraindications
Hypersensitivity to any component of this product.
Special Warnings and Special Precautions for Use
Local Irritation
It is not recommended to initiate treatment with RETINO-A® or continue its use in the presence of skin irritation (e.g., erythema, peeling, pruritus, sunburn, etc.) until these symptoms subside.
In certain sensitive individuals, RETINO-A® may induce severe local erythema, swelling, pruritus, warmth, burning or stinging, blistering, crusting and/or peeling at the site of application. If the degree of local irritation warrants, the patient should be instructed to either apply the medication less frequently or discontinue its use temporarily.
Tretinoin has been reported to cause severe irritation on eczematous skin and should be used with utmost caution in patients with this condition. If a patient experiences severe or persistent irritation, the patient should be advised to discontinue application of Retin-a® completely, and if necessary, consult a physician.
In order to minimize the potential for additional skin irritation, RETINO-A® should be kept away from the eyes, the mouth, paranasal creases of the nose, and mucous membranes or other areas where treatment is not intended.
Weather extremes, such as wind, cold and low humidity may be irritating to skin treated with RETINO-A® and may increase its dryness.
Patients will be able to remove hair as usual (e.g. plucking, electrolysis, depilatories) but should avoid these procedures at night before applying Retin-a cream as they might result in skin irritation.
Permanent wave solutions, waxing preparations, medicated soaps and shampoos can sometimes irritate even normal skin. Caution should be used so that these products do not come into contact with skin treated with Retin-a.
Exposure to Sunlight
Exposure to sunlight, including ultraviolet sunlamps, may provoke additional irritation. Therefore, exposure should be avoided or minimized during the use of tretinoin. Patients with sunburn should be advised not to use the product until fully recovered because of potential severe irritation to sensitive skin. Patients who may be required to have considerable sun exposure due to their occupation, and those with inherent sensitivity to the sun, should exercise particular caution. When exposure to sunlight cannot be avoided, use of sunscreen products and protective clothing over treated areas is recommended.
Interactions with Other Medicinal Products and Other Forms of Interaction
The following products or medications should be used with caution because of possible interaction with tretinoin. It is advised to allow the effects of such preparations to subside before use of RETINO-A® is begun:
- Concomitant topical medication;
- Preparations containing benzoyl peroxide, sulfur, resorcinol, or salicylic acid;
- Toiletry preparations having an abrasive, drying, or desquamative effect, including soaps, shampoos, cosmetics, and products with high concentrations of alcohol, astringents, spices or lime
Pregnancy and Lactation
Use during pregnancy
Topical tretinoin has not been shown to be teratogenic in Wistar rats and rabbits when given in doses 1000 and 320 times the topical human dose, respectively, assuming that a 50 kg adult applies 250 mg of 0.1% RETINO-A® cream topically. At these topical doses, however, a delayed ossification of several bones occurred in rabbits. In rats, a dose-dependent increase of supernumerary ribs was observed. These changes are considered variants of normal development. The ossification changes are usually spontaneously corrected after weaning.
There have been isolated reports of birth defects among babies born to women exposed to topical tretinoin during pregnancy. To date, there have been no adequate and well-controlled studies performed in pregnant women, and the teratogenic blood level of tretinoin is not known. However, a well-conducted retrospective cohort study of babies bom to women exposed to topical tretinoin during the first trimester of pregnancy found no excess birth defects among these babies when compared to babies born to women in the same cohort who were not similarly exposed. Nevertheless, topical tretinoin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (see section on Preclinical safety data).
[Oral tretinoin has been shown to be teratogenic and fetotoxic in rats when given in doses 2000 and 500 times the topical human dose, respectively.]
Use during lactation
It is not known whether this drug is excreted in human milk. Since many drugs are excreted in human milk, caution should be exercised when Retin-a cream® is administered to a nursing woman.
Effects on Ability to Drive and Use Machines
Use of RETINO-A® is not known to affect the ability to drive a motor vehicle or operate machinery.
Undesirable Effects
Clinical Trial Data
The safety of tretinoin topical formulations including RETINO-A® was evaluated in 4160 patients (of whom 3035 were treated with topical tretinoin and 1125 received placebo) who participated in 23 clinical trials, including 4 open-label and 19 double-blind, placebo-controlled clinical trials. The 23 clinical trials evaluated the safety of tretinoin in male and female patients, 10 to 79 years of age with photodamaged skin or acne vulgaris.
Adverse drug reactions (ADRs) reported for >=1% of tretinoin-treated patients in 19 double-blind, placebo-controlled clinical trials are shown in Table 1.
Table 1. Adverse Drug Reactions Reported by >=1% of Tretinoin- Treated Patients in 19 Double-Blind, Placebo-Controlled Clinical Trials of Tretinoin
|
System/Organ Class Adverse Reaction |
Tretinoin % (N=1701) |
Placebo (N=1125) |
|
Skin and Subcutaneous Tissue Disorders Hyperkeratosis |
12.2 |
4.5 |
|
Skin irritation |
10.7 |
8.5 |
|
Pain of skin |
10.1 |
3.4 |
|
Erythema |
8.0 |
3.6 |
|
Pruritus |
5.9 |
3.0 |
|
Rash popular |
5.7 |
4.5 |
|
Rash |
3.8 |
2.4 |
|
Dermatitis |
2.7 |
1.7 |
|
Dry skin |
2.7 |
0.7 |
|
Skin exfoliation |
2.4 |
2.6 |
|
Nervous System Disorders Headache |
3.5 |
4.7 |
Adverse drug reactions reported by <1% of tretinoin-treated patients (N=3035) in 23 clinical trials are shown in Table 2.
Table 2. Adverse Drug Reactions Reported by <1% of Tretinoin-Treated Patients in 23 Clinical Trials of Tretinoin
|
System/Organ Class Adverse Reaction |
|
Skin and Subcutaneous Tissue Disorders |
|
Swelling face |
|
Blister |
|
Skin discolouration |
|
Skin hyperpigmentation |
|
Skin hypopigmentation |
|
Skin burning sensation |
|
General Disorders and Administration Site Conditions |
|
Feeling hot |
Postmarketing Experience
Adverse drug reactions first identified during postmarketing experience with tretinoin are included in Table 3. In the table, the frequencies are provided according to the following convention :
Very common >= 1/10
Common >= 1/100 and < 1/10
Uncommon >= 1/1,000 and <1/100
Rare >= 1/10,000. <1/1,000
Very rare <1/10,000, including isolated reports
In Table 3, ADRs are presented by frequency category based on spontaneous reporting rates, when known.
Table 3. Adverse Drug Reactions Identified During Postmarketing Experience with Tretinoin by Frequency Category Estimated from Spontaneous Reporting Rates
|
Immune System Disorders |
|
Very rare Hypersensitivity |
|
Eye disorders |
|
Very rare Eye irritation |
|
Skin and Subcutaneous Tissue Disorders |
|
Very rare Photosensitivity reaction, Scab, Urticaria |
Overdose
RETINO-A® is intended for topical use only. Excessive application of RETINO-A® does not improve the results of treatment and may induce marked irritation, e.g., erythema, peeling, pruritus, etc. Oral ingestion of large amounts of the drug may lead to the same side effects as those associated with excessive oral intake of Vitamin A (e.g., pruritus, dry skin, arthralgias, anorexia, vomiting). In the event of accidental ingestion, if the ingestion is recent, the stomach should be emptied immediately by gastric lavage or by induction of emesis. All other treatment should be appropriately supportive.
PHARMACOLOGICAL PROPERTIES
Tretinoin, all-trans-retinoic acid, is a derivative of vitamin A.
Pharmacodynamic Properties
Pharmacotherapeutic group (АТС code): D10AD01
Although the exact mode of action of tretinoin is unknown, current evidence suggests that the effectiveness of tretinoin in acne is due primarily to its ability to modify abnormal follicular keratinization. Comedones form in follicles with an excess of keratinized epithelial cells. Tretinoin promotes detachment of cornified cells and the enhanced shedding of corneocytes from the follicle. By increasing the mitotic activity of follicular epithelia, tretinoin also increases the turnover rates of thin, loosely-adherent corneocytes. Through these actions, the comedone contents are extruded and the formation of the microcomedo, the precursor lesion of acne vulgaris, is reduced.
Additionally, tretinoin acts by modulating the proliferation and differentiation of epidermal cells. These effects are mediated by tretinoin's interaction with a family of nuclear retinoic acid receptors. Activation of these nuclear receptors causes changes in gene expression. The exact mechanisms whereby tretinoin-induced changes in gene expression regulate skin function are not understood.
Pharmacokinetic Properties
Absorption
Tretinoin is an endogenous metabolite of Vitamin A metabolism in man. Upon topical application, tretinoin is minimally absorbed, penetrating both the epidermis and dermis.
Percutaneous absorption of tretinoin, as determined by the cumulative excretion of radiolabeled drug into urine and feces, was assessed in healthy men and women after single and/or repeated daily applications of a 0.05%, 0.1% or 0.5% tretinoin cream formulation, at doses of 100, 150 or 500 mg. The mean percutaneous absorption ranged from 1.0 to 4.3%. Endogenous plasma concentrations of tretinoin and its metabolites, 13-cis-retinoic acid, all-trans-4-oxo-retinoic acid and 13-cis-4-oxo-retinoic acid were essentially unaltered after either single or multiple daily applications relative to baseline levels.
Distribution
Approximately 80% of tretinoin applied remains on the skin surface, whereas its penetration through the stratum corneum and the hair follicle is vehicle-dependent. After the initial diffusion into the stratum corneum that occurs within a few minutes, further diffusion into epidermis and dermis proceeds more slowly.
Metabolism
Topically-applied tretinoin is metabolized by CYP2S1 and CYP26. Metabolites are 13-cis-retinoic acid, all-trans-4-oxo-retinoic acid and 13-cis-4-oxo-retinoic acid.
Elimination
After application of radiolabeled tretinoin emollient cream or cream, urinary excretion occurred mainly in the first 48 hours, whereas radioactivity was eliminated in the feces throughout the 7 days after dose application. On average 1 -1.5% of the radioactivity was recovered in urine and less than 1 % was recovered in feces.
Paediatric Population
It is expected that pharmacokinetic behavior of tretinoin topical formulations and drug-drug interactions with tretinoin topical formulations will be similar to those in adults.
Preclinical Safety Data
General Toxicity
Preclinical safety studies showed no acute signs of toxicity in rats receiving up to 2500 mg/kg as a single oral dose.
A subchronic study (28 days) in rabbits and a chronic study (91 weeks) in mice treated with topical application of tretinoin produced typical changes associated with retinoids including alopecia, scaling, edema, flaccid skin tone, erythema, eschar, ulceration, epidermal hyperplasia and acanthosis.
Most tretinoin formulations were mild to moderate dermal irritants on the skin of mice and rabbits. In the rabbit eye, they were minimal or non-irritants. In the guinea pig, they were non-sensitizers.
Carcinogenicity
In a life-time topical study of tretinoin in CD-1 mice, there was no evidence of carcinogenic potential.
Studies in hairless albino mice suggest that tretinoin may accelerate the tumorigenic potential of carcinogenic light from a solar simulator. In other studies, when lightly pigmented hairless mice treated with tretinoin were exposed to carcinogenic doses of UVB light, the incidence and rate of development of skin tumors was reduced. Due to significantly different experimental conditions, no strict comparison of these data is possible. Although the significance of these studies to man is not clear, patients should avoid or minimize exposure to sun.
Mutagenicity
Tretinoin had no mutagenicity in an in vivo mouse micronucleus assay. The mutagenic potential of tretinoin was also evaluated in the Ames assay which was also negative.
Reproduction / Teratology
Orally administered tretinoin during pregnancy produces dose-dependent and stage-dependent fetal anomalies in several species. In Segment II oral and dermal teratology studies in Wistar rats, frank fetal malformations were observed only after oral administration of 10 mg/kg tretinoin where one fetus in each of 3 litters showed cleft palate. No fetal malformations resulted after oral or dermal application of tretinoin at 1, 2.5, or 5 mg/kg doses. Oral and dermal doses of > 2.5 mg/kg tretinoin produced an increased incidence of fetuses with skeletal variations (greater in oral), e.g., vestigial ribs. Skeletal variations, while treatment-related, are not categorized as teratogenic outcomes, but as segmental variations of embryonic pattern formation, and as such are not incompatible with normal development. While oral tretinoin produced a higher incidence of fetal effects than dermal tretinoin, the overall fetal no-observable-effect-level by either dosage route is 1 mg/kg. The findings in the two above-mentioned studies are consistent with results reported from numerous earlier studies.
Pharmaceutical particulars
List of Excipients :
Polyoxyl 40 Stearate USNF, Stearyl Alcohol IP, Isopropyl Myristate BP, Butylated hydroxy toluene IP, Stearic Acid IP, Sorbic Acid IP, Xanthan Gum ' BP, Purified water.
Shelf Life:
See on pack
Special Precautions for Storage:
Store below 25°C, protected from light. Do not Freeze. Replace Cap immediately.
Keep out of reach and sight of Children.
| FOR EXTERNAL USE ONLY |
Warning: To be sold by retail on the prescription of a Registered Medical Practitioner
Presentation :
20 g Aluminium collapsible tube
DATE OF REVISION OF THE TEXT
Oct 2016.
 Reference : Company Core Data Sheet (CCDS) dated 21 May 2012.
Reference : Company Core Data Sheet (CCDS) dated 21 May 2012.
Manufactured by:
Encube Ethicals Pvt. Ltd.,
Plot No. C1, Madkaim Ind. Estate,
Madkaim, Post Mardol, Ponda,
Goa - 403 404, India.
Marketed by:
Johnson & Johnson Private Limited, L.B.S. Marg, Mulund (West), Mumbai 400 080.
©Registered Trademark of Johnson & Johnson U.S.A.








