No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
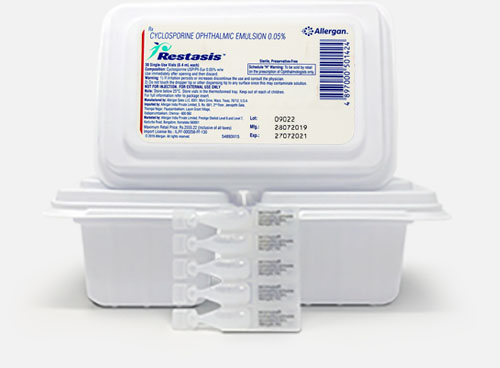
Restasis eye drops 0.05% 0.4ml
New
295 Items
More info

|
Basic Information
Not for injections. For external use only! |
 |
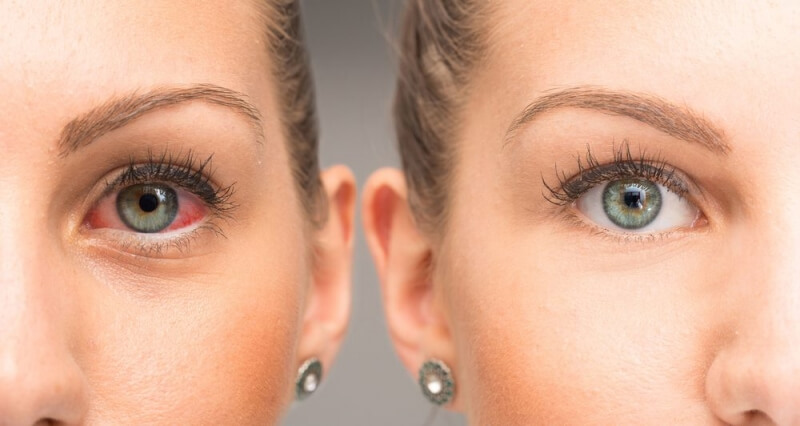
Are you still troubled by Chronic Dry Eye Disease?
Restasis containing cyclosporine is a unique product on the market that focuses on the root of the problem. The biggest advantage
of the product as it makes your natural tears to produce, unlike other products that work like artificial tears.
As Dry Eye disease develops over time, it will take some time for Restasis to start working. The good treatment results are usually achieved within a 6-months period. After that period people can produce much more of their own tears. Chronic Dry Eye Disease is a chronic condition characterized by redness, itching, sensitivity to lights, burning. 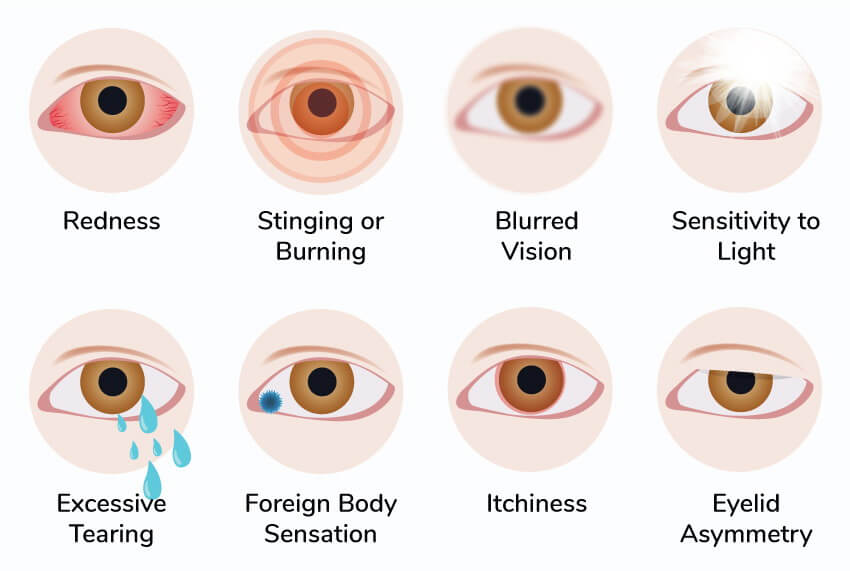
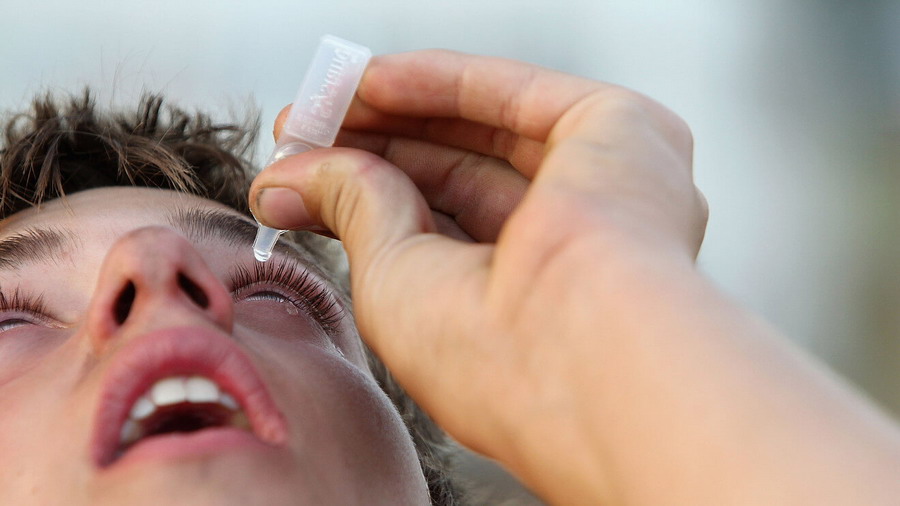
How to use Restasis single-dose vials?
|
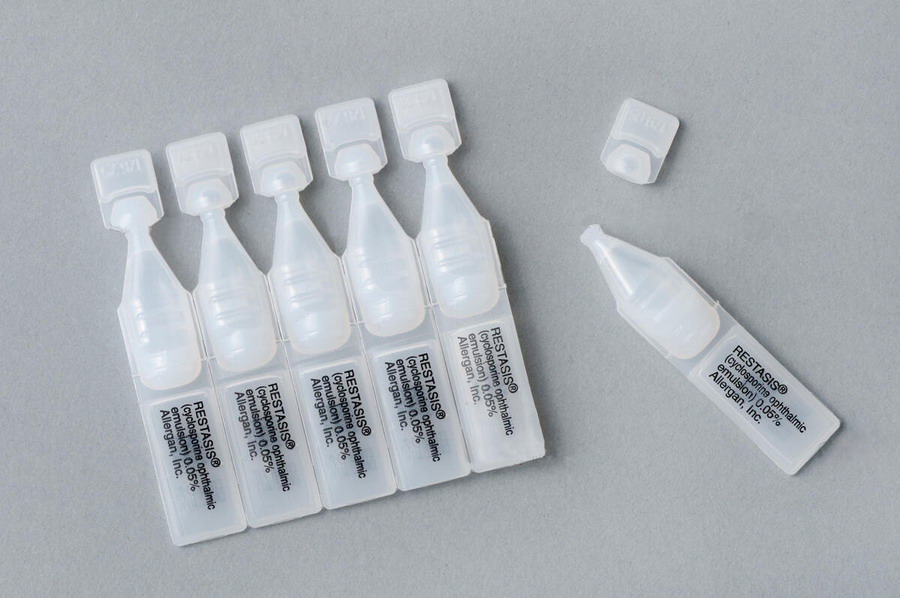
One pack contains 30 single-dose vials equivalent to 15 days of treatment.
|
 |
What to expect from Restasis Single-use vials?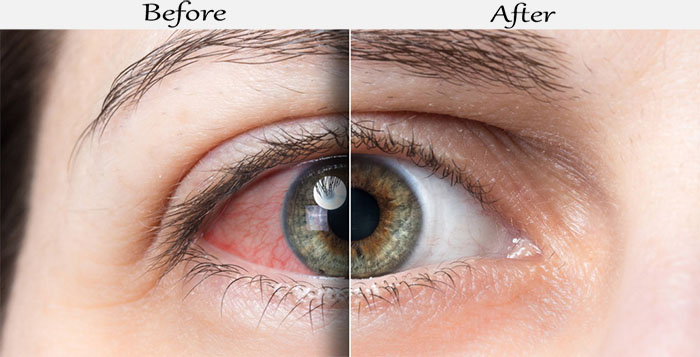
|
Why do you need to use Restasis? |
||||
|
1.Original product by Allergan, USA
2.A unique product that restores the ability to produce your own tears.
3.Great results are achieved within 6 months.
4.Super Price directly from the manufacturer.
5.Clinically proven results.
|
 |
|||
How does Restasis work?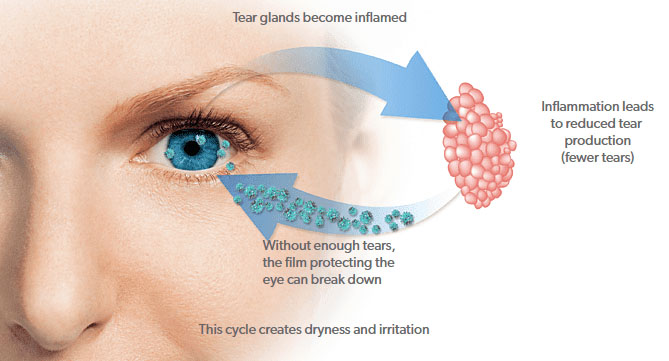
Restasis containing Cyclosporine is much different from artificial tears. Cyclosporine works by suppressing the local immune response and this way decreases inflammatory reaction. By suppressing the inflammatory reaction, Restasis helps to restore normal tear production.
Restasis monthly progress!
- 1st Month - Your eyes may begin to produce more real tears. The desired effect is still not achieved.
- 3d Month - Most people notice much more tears to produce. It is important to continue using Restasis.
- 6th Month - A significant increase in tears production is observed. During this period is it important to evaluate progress with your doctor.
Chronic Dry Eye disease is a long-term disease and can not be cured at once. Patience and strict adherence to the rules are important to get rid of the problem.
Congratulations! You decided to start your journey with Restasis. Natural tears matters for your eye.
Restasis eye drops brief description
Product name - Restasis eye drops 0.05% brand
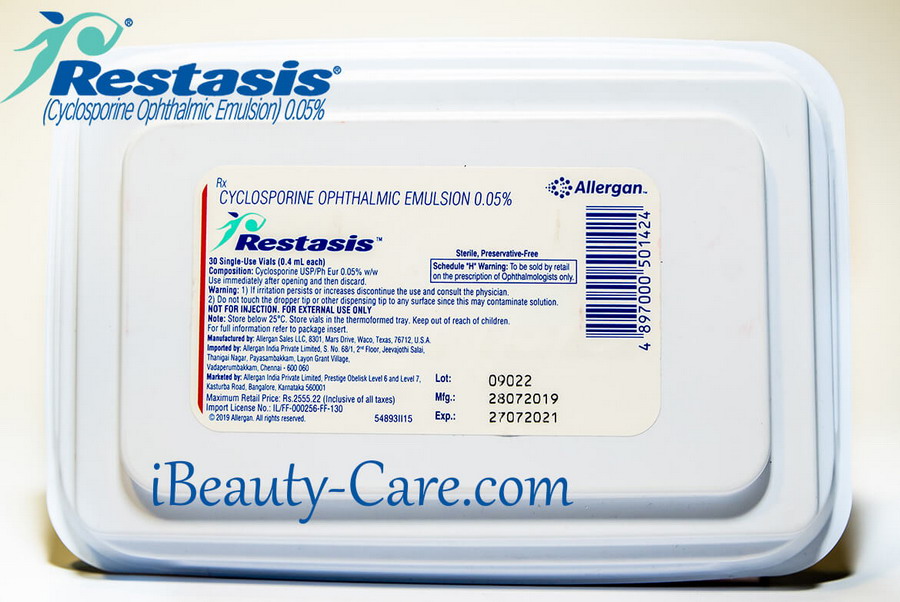
Qualitative and quantitative composition - Each mL of sterile, preservative-free ophthalmic emulsion contains 0.5 mg of cyclosporine. Other ingredients include Type A copolymer carbomer, purified water, glycerin, castor oil and sodium hydroxide to adjust pH. One pack contains 30 single-use vials.
Action mechanism - The local application of Restasis (cyclosporine) makes it possible to reduce the inflammatory response of the eye and offer comfort. In some people, it can make the eye normal. If the dryness of the eye is due to the inflammatory response of the immune cells then it can be controlled by cyclosporine as the drug suppresses the immune response. Cyclosporine eye drops are thought to target the immune cells of the eyes to reduce the inflammation that appears to be the cause of dry eye. It should be noted that cyclosporine is not a medicine for dry eyes. It has been used in the medical world as an immunosuppressant and is also used to tackle autoimmune disorders. It has been realized that your dry eye disorder could be caused by T cells, a type of cells of the immune system. Restasis (Cyclosporine) helps in suppressing the immune response and thus the dryness of the eye. Remember, it cannot control the dry eye caused by other conditions such as vitamin A deficiency or scarring. Therefore, it should not be used in these scenarios.
Indication - Restasis is indicated to people with dry eye or need to control inflammation of the eye or any such conditions that requires suppression of immune response.
Mode of administration - The recommended dose of ophthalmic drops of Restasis (cyclosporine) is 1 drop in the affected eye, 2 times a day, about 12 hours apart. Ophthalmic drops of cyclosporine are presented as a single-use vial in a package to be closed. Before using this medication, gently shake the vial several times, so that the emulsion becomes uniformly white throughout the vial. Instill the medication immediately after opening the single-use vial and discard it immediately after use.
You should avoid the top of the bottle touching the eye or any other surface so that the medicine is not contaminated. Do not touch your eye with the bottle to avoid hurting yourself.
If you also use a solution of artificial tears, wait 15 minutes between the use of ophthalmic drops of cyclosporine and that of the solution of artificial tears.
Contraindication - Restasis (cyclosporine ophthalmic drops) should not be used in the following circumstances:
-
an allergy to cyclosporine eye drops or any ingredients of the medication
-
an active eye infection
Special cautions and warnings - Restasis Ophthalmic drops of cyclosporine can temporarily cause blurred vision. Do not drive or do dangerous work or use heavy machines until you are sure your vision is clear and this medicine does not affect your ability to perform these tasks safely.
The effect of Restasis cyclosporine eye drops has not been studied in people with a history of herpetic keratitis, terminal tear gas disease, dry eye due to vitamin A deficiency or scarring. If you have any of these disorders, consult your doctor.
This eye drop should not be applied at the time of pregnancy unless your doctor suggests it. If pregnancy occurs while you use this medicine, contact your doctor immediately.
It is not known if ophthalmic cyclosporine passes into breast milk. If you take this medicine while you are breastfeeding, your baby may feel the effects. Consult your doctor to find out if you should continue breastfeeding or take the medicine.
The drug is not suitable or recommended for children as its effectiveness, as well as the safety, is not confirmed for children.
Drug interaction - No specific drug interaction is known. Tell your doctor everything you are taking, whether prescription or over-the-counter medications and herbal remedies. Do not forget to mention any supplements you take.
Side effects - A Restasis side effect is an undesirable response to a drug when taken at normal doses. Some of the common side effects of Restasis eye drops are the impression of having something in the eye, sensitivity to light, redness of the eyes, pain in the eyes, headaches, eye irritation and itchy eye, eye discharge, burning sensation in the eyes, blurred vision, etc.
Some rare side effects such as hives, swelling of the eye, and abnormalities of the visual field may occur in a few individuals.
Interesting facts
-
Store this medicine at a temperature between 15°C and 25°C and keep it out of the reach of children.
-
Do not dispose of medicines in wastewater (e.g., in the sink or in the toilet bowl) or in household waste. Ask your pharmacist about how to get rid of unused or expired medications.
-
Do not use the medicine if you are experiencing any type of allergic reactions
-
This medicine is not suitable for children and infants
Generic Latisse Real Shots
Full Original Product Annotation
 Restasis eye drops
Restasis eye drops
CONTENTS
- Carcinogenesis, Mutagenesis, and Impairment of Fertility:
- Teratogenic effects:
- Non-Teratogenic effects:
USE IN PREGNANCY AND LACTATION
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
DESCRIPTION
RESTASIS™ (cyclosporine ophthalmic emulsion) 0.05% contains a topical immunomodulator with anti-inflammatory effects.
Cyclosporine's chemical name is Cyclo[[(E)-(2S,3R,4R)-3-hydroxy-4-methyl-
2-(methylamino)-6-octenoyl]-L-2-aminobutyryl-N-methylglycyl-N-methyl-
L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-
N-methyl-L-leucyl-N-methyl-L-valyl] and it has the following structure:
Restasis Structural Formula
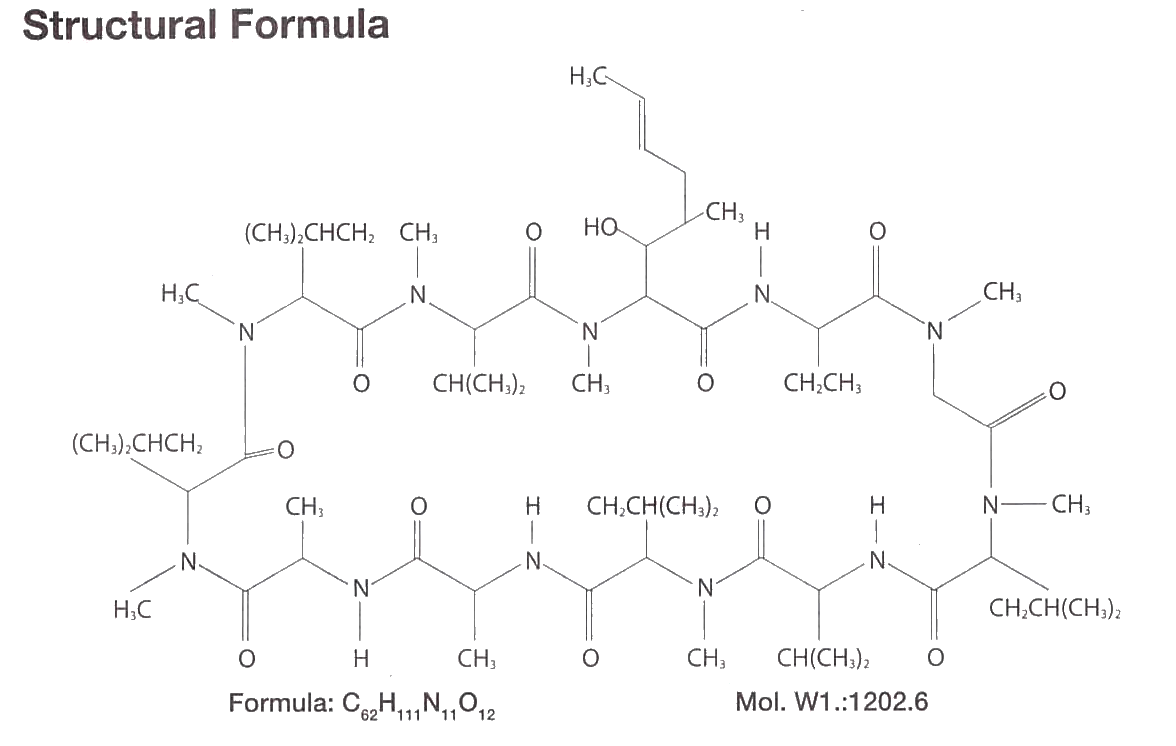
Cyclosporine is a fine white powder. RESTASIS™ appears as a white opaque to slightly translucent homogeneous emulsion. It has an osmolality of 230 to 320 mOsmol/kg and a pH of 6.5-8.0.
Each mL of RESTASIS™ contains: Active: cyclosporine 0.05%. Inactive: glycerin; castor oil; polysorbate 80; carbomer 1342; purified water and sodium hydroxide to adjust the pH.
CLINICAL PHARMACOLOGY
Mechanism of action:
Keratoconjunctivitis sicca (chronic dry eye disease) is a syndrome of multiple aetiologies, which have in common an immune-based inflammation of the lachrymal glands and ocular surface.
Topical cyclosporine emulsion achieves its immunomodulatory/anti-inflammatory activity by inhibiting the activation of NF-kВ, a nuclear factor involved in the regulation of immune and pro-inflammatory cytokine response genes, such as TNF, IL-1, IL-2, and IL-8. It prevents the synthesis and/or secretion of several TH1 pro-inflammatory cytokines such as IL-2, IL-6, IFN-y, IL-8, and TNF-a. It is also known to upregulate secretion of TH2-type anti-inflammatory cytokines, including IL-13. IL-13 is thought to be one of the pivotal proteins involved in regulating TH2 (anti-inflammatory cytokine) production. Although cyclosporine affects a number of immune mechanisms it leaves critical functions of host immunity intact. Challenges to the ocular surface can still be met with T cells as well as Вcells, phagocytes and other immune-responsive cells. Supportive evidence for the immune integrity of the ocular surface is demonstrated by the lack of opportunistic ocular infections found in animals and humans.
Blood cyclosporin A concentrations were measured using a specific high pressure liquid chromatography-mass spectrometry assay. Blood concentrations of cyclosporine, in all the samples collected, after topical administration of Restasis eye drops 0.05%, BID, in humans for up to 12 months, were below the quantitation limit of 0.1 ng/mL. There was no detectable drug accumulation in blood during 12 months of treatment with RESTASIS™.
Four multicenter, randomized, adequate and well-controlled clinical studies were performed in approximately 1200 patients with moderate to severe keratoconjunctivitis sicca. RESTASIS™ demonstrated statistically significant increases in Schirmer wetting of 10 mm versus vehicle at six months in patients whose tear production was presumed to be suppressed due to ocular inflammation. This effect was seen in approximately 15% of RESTASIS™ treated patients versus approximately 5% of vehicle treated patients. Increased tear production was not seen in patients currently taking topical anti-inflammatory drugs or using punctal plugs. No increase in bacterial or fungal ocular infections was reported following administration of RESTASIS™.
RESTASIS™ is indicated to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca. Increased tear production was not seen in patients currently taking topical anti-inflammatory drugs or using punctal plugs.
RESTASIS™ is contraindicated in patients with active ocular infections and in patients with known or suspected hypersensitivity to any of the ingredients in the formulation.
RESTASIS™ has not been studied in patients with a history of herpes keratitis.
PRECAUTIONS
General: For ophthalmic use only
Information for Patients: The emulsion from one individual single-use vial is to be used immediately after opening for administration to one or both eyes, and the remaining contents should be discarded immediately after administration. To avoid contamination or possible eye injury, do not touch tip of the bottle or vial to any surface and avoid contact with the eye. Restasis eye drops should not be administered while wearing contact lenses. Patients with decreased tear production typically should not wear contact lenses. If contact lenses are worn, they should be removed prior to the administration of the emulsion. Lenses may be reinserted 15 minutes following administration of RESTASIS™.
DRUG INTERACTIONS
No interaction studies have been performed.
Drugs that affect the cytochrome P-450 ЗА may alter cyclosporine metabolism. There is no detectable systemic absorption of RESTASIS™ following ocular administration. Therefore, no interaction of topically applied RESTASIS™ with systemic drugs is expected to occur.
PRECLINICAL SAFETY DATA
Carcinogenesis, Mutagenesis, and Impairment of Fertility: Systemic carcinogenicity studies were carried out in male and female mice and rats. In the 78-week oral (diet) mouse study, at doses of 1, 4 and 16 mg/kg/day, evidence of a statistically significant trend was found for lymphocytic lymphomas in females, and the incidence of hepatocellular carcinomas in mid-dose males significantly exceeded the control value.
In the 24-month oral (diet) rat study, conducted at 0.5, 2, and 8 mg/kg/day, pancreatic islet cell adenomas significantly exceeded the control rate in the low dose level. The hepatocellular carcinomas and pancreatic islet cell adenomas were not dose related. The low doses in mice and rats are approximately 1000 and 500 times greater, respectively, than the daily human dose of one drop (28 µl) of 0.05% RESTASIS™ BID into each eye of a 60kg person (0.001 mg/kg/day), assuming that the entire dose is absorbed.
Cyclosporine has not been found mutagenic/genotoxic in the Ames Test, the V79-HGPRT Test, the micronucleus test in mice and Chinese hamsters, the chromosome-aberration tests in Chinese hamster bone-marrow, the mouse dominant lethal assay, and the DNA-repair test in sperm from treated mice. A study analyzing sister chromatid exchange (SCE) induction by cyclosporine using human lymphocytes in vitro gave indication of a positive effect (i.e. induction of SCE).
No impairment in fertility was demonstrated in studies in male and female rats receiving oral doses of cyclosporine up to 15 mg/kg/day (approximately 15,000 times the human daily dose of 0.001 mg/kg/day) for 9 weeks (male) and 2 weeks (female) prior to mating.
Teratogenic effects: No evidence of teratogenicity was observed in rats or rabbits receiving oral doses of cyclosporine up to 300 mg/kg/day during organogenesis. These doses in rats and rabbits are approximately 300,000 times greater than the daily human dose of one drop (28 pl) 0.05% RESTASIS™ BID into each eye of a 60kg person (0.001 mg/kg/day), assuming that the entire dose is absorbed.
Non-Teratogenic effects: Adverse effects were seen in reproduction studies in rats and rabbits only at dose levels toxic to dams. At toxic doses (rats at 30 mg/kg/day and rabbits at 100 mg/kg/day), cyclosporine oral solution, USP, was embryo and fetotoxic as indicated by increased pre- and postnatal mortality and reduced fetal weight together with related skeletal retardations. These doses are 30,000 and 100,000 times greater, respectively, than the daily human dose of one drop (28 µl) of 0.05% RESTASIS™ BID into each eye of a 60 kg person (0.001 mg/kg/day), assuming that the entire dose is absorbed
No evidence of embryo fetal toxicity was observed in rats or rabbits receiving cyclosporine at oral doses up to 17 mg/kg/day or 30 mg/kg/day, respectively, during organogenesis. These doses in rats and rabbits are approximately 17,000 and 30,000 times greater, respectively, than the daily human dose. Offspring of rats receiving a 45 mg/kg/day oral dose of cyclosporine from Day 15 of pregnancy until Day 21 post partum, a maternally toxic level, exhibited an increase in postnatal mortality; this dose is 45,000 times greater than the daily human topical dose, 0.001 mg/kg/day, assuming that the entire dose is absorbed. No adverse events were observed at oral doses up to 15 mg/kg/day (15,000 times greater than the daily human dose).
USE IN PREGNANCY AND LACTATION
There are no adequate data from the use of RESTASIS™ in pregnant women. Cyclosporine ophthalmic emulsion 0.05% is not absorbed systemically following topical ocular administration, and maternal use is not expected to result in foetal exposure to the drug. Studies in animals have shown reproductive toxicity only at high maternotoxic doses. RESTASIS™ should be administered to pregnant women only if clearly needed.
Cyclosporine is known to be excreted in human milk following systematic administration but excretion in milk after topical treatment has not been investigated. Although blood concentrations are undetectable after topical administration of RESTASIS™, caution should be exercised when RESTASIS™ is administered to a nursing woman.
PEDIATRIC USE
The safety and efficacy of RESTASIS™ has only been studied in adults.
GERIATRIC USE
No overall difference in safety or effectiveness has been observed between elderly and younger patients.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
RESTASIS™ may cause transient blurring of vision which may impair the ability to drive or operate machines. The patient should wait until their vision has cleared before driving or using machinery.
ADVERSE REACTIONS
On combining the data from the pivotal phase 3 clinical studies, approximately 29% of treated patients experienced treatment related adverse events (adverse reactions) in the first year. The majority were ocular, mild or moderate in severity and none were serious. The most common adverse event following the use of RESTASIS™ was eye burning reported in approximately 17% of patients in the first year with the incidence of new reports decreasing to 5% at 2 years.
Other events commonly reported in 1 % to 10% of patients included ocular /conjunctival hyperemia, headache, eye irritation, eye discharge, epiphora, eye pain, foreign body sensation, eye pruritus, eye stinging, photophobia and visual disturbance (blurred vision), dry eye.
POSTMARKETING EXPERIENCE
The following additional adverse reactions have been identified during postmarketing use of RESTASIS™ in clinical practice. Because postmarketing reporting of these reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions.
Adverse Events including the following:
Eye swelling, burning sensation, Pruritus, Urticaria, Hypersensitivity, Rare cases including severe angioedema, face swelling, tongue swelling, pharyngeal edema, and dyspnea, Superficial injury of the eye (from the vial tip touching the eye during administration)
DOSAGE AND ADMINISTRATION
Invert the unit dose vial a few times to obtain a uniform, white, opaque emulsion before using. Instill one drop of RESTASIS™ twice a day in each eye approximately 12 hours apart. RESTASIS™ can be used concomitantly with another topical ophthalmic product, allowing a 15 minute interval between products. Discard vial immediately after use. Patients should be advised not to discontinue therapy prematurely.
Systemic overdose is unlikely to occur after ocular administration of RESTASIS™. Due to low systemic concentrations of cyclosporine after topical treatment with RESTASIS™, systemic intoxication from topical overdose in humans is not expected.
Restasis eye drops is packaged in single use vials. Each vial contains 0.4 mL fill in a 0.9 mL LDPE vial; 30 vials are packaged in a polypropylene tray with an aluminium peelable lid.
STORAGE
Store RESTASIS™ below 25°C.
KEEP OUT OF REACH OF CHILDREN.
Rx Only
Revision date: Oct 2017
Manufactured by:
Allergan Sales, LLC Waco, Texas, U.S.A.
Imported by:
Allergan India Private Limited
S. No. 68/1,2nd Floor, Jeevajothi Salai,
Thanigai Nagar, Payasambakkam,
Layon Grant Village, Vadaperumbakkam,
Chennai - 600 060
© 2018 Allergan. All rights reserved.
All trademarks are the property of their respective owners.