No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
Prilox cream 2.5% 5g (Generic Emla)
New
Two local anesthetics, prilocaine, and lidocaine (both amide type) are present in Prilox cream and they act to reduce pain sensation to the applied area. The cream is primarily used to prevent pain and as an anesthetic to various external areas of the human body - such as the genital area. It is applied only to unbroken and normal skin areas.
298 Items
More info


|
Basic Information
|
 |
Prilox (generic Emla) cream - best local anesthesia available!
Are you still looking for local anesthesia without side effects?
The good news is that generic Emla known as Prilox cream is available now. Prilox is a clinically approved cream for local anesthesia used before different medical procedures like needles insertions, vaccinations, superficial surgical procedures, cosmetic procedures such as a tattoo.
Try Prilox Cream for local anesthesia!
Prilox cream is a combination of two anesthetics that help to numb the area where they are applied. Generic Emla cream (prilox) can significantly reduce pain sensation during cosmetic procedures for example tattoo removal or bikini waxing.
It is intended only for external use. Do not apply to open wounds. 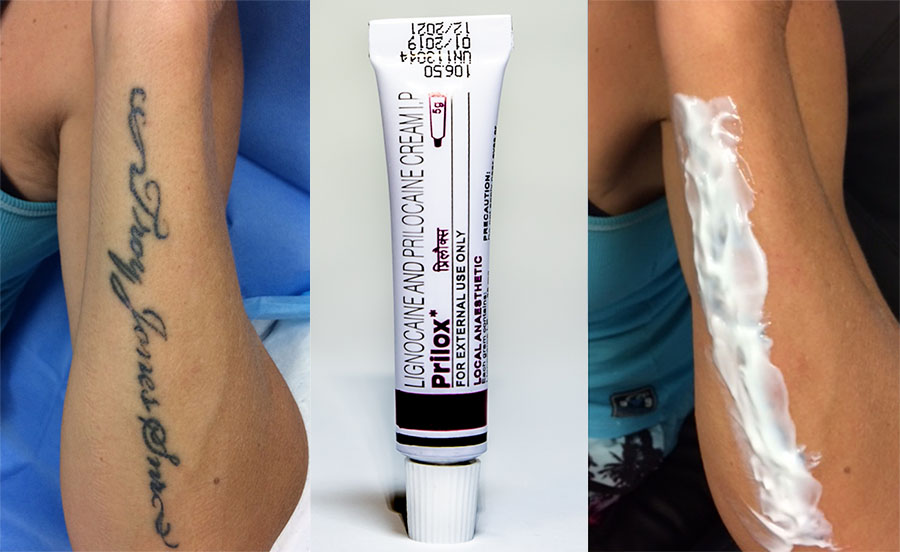
|
Why do you need to use generic Emla cream (Prilox cream)? |
||||
|
 |
|||
How Long does it take for Prilox cream to work?
Time taking for Prilox to start working depends on many factors such as applied area, skin condition, age, and type of
a procedure intended for use.
For most procedures apply Prilox cream 1 hour before procedure except for:
-
Male genital 15 minutes before the procedure
- Leg ulcer cleaning - 30 minutes before the procedure
- Skin grafting - 120 minutes before the procedure
- Genital mucosa - 10-15 minutes before the procedure
-
Cervical curettage - 10-15 minutes before the procedure
Full satisfaction is guaranteed!!!
Emla cream and Prilox cream are identical products made by two different manufacturers.
They contain the same active ingredients Lignocaine and Prilocaine and are used for the same purposes. Both products provide great results for local anesthesia (to numb skin areas before different medical and cosmetic procedures)
- Emla=Prilox
- Same active ingredients
- Same additives
- Same volume
- Same indications
- Same results for less money

Generic Emla cream (prilox) brief description
Product name - Prilox Cream 2.5% 5g (generic Emla)
For external use only! Local anesthetic.
Qualitative and quantitative composition - Prilox Cream is a local anesthetic cream and a type of combination medicine. 1 gram of the cream contains equal amount of prilocaine and lidocaine (25 mg). Other ingredients are Macrogolglycerol hydroxystearate, Purified water, Sodium hydroxide, and Carbomer.
Action mechanism - Generic Emla has two active components prilocaine and lidocaine. Both these compounds act to reduce the feeling of pain by suppressing pain signals to the brain. This decreases the feeling of pain. Lidocaine and prilocaine are local amide anesthetics. These two components stabilize the neuronal membranes by inhibiting the ionic flux necessary for the initiation and the conduction of the influxes, thus producing local anesthesia. The quality of the anesthesia depends on the application time and the dose used. It is a good option to avoid pain before several procedures like laser surgery of the skin, skin grafts, or needle insertion for different reasons and treatments, etc. The cream acts by numbing the area of application for a certain period.
Indication - Prilox cream is indicated when local anesthesia is needed through topical application. For example, the insertion of needles, during intravenous catheters or drawing blood samples; superficial surgical procedures; topical anesthesia of the genital mucosa, for example before superficial surgical procedures or infiltration anesthesia; in adults and adolescents ≥ 12 years old; topical anesthesia in leg ulcer treatments to facilitate mechanical cleaning/debridement in adults only.
Mode of administration - One gram of Prilox cream corresponds to approximately 3.5 cm length of cream taken from a 30 g tube. A thick layer of Prilox cream should be applied to the skin, including the skin of the genitals, under an occlusive dressing. For application over larger areas, such as for thin skin grafting, an elastic bandage should be applied over the occlusive dressing to ensure even distribution of the cream and protect the area. In the presence of atopic dermatitis, the duration of application should be reduced. The dose depends on the reason or type of condition or procedure for which local anesthesia is needed.
Contraindication - Your doctor will avoid using Prilox cream in the following conditions:
- Deficit in G6PD
- Genital mucosa of the child
- Hypersensitivity polyoxyethylene castor oil
- Hypersensitivity prilocaine
- Injured tympanum
- Injury open
- Lidocaine hypersensitivity
- Local anesthetic hypersensitivity of the amide group
- methemoglobinemia
- Premature infants less than 37 weeks
Special cautions and warnings - Prilox cream should not be used in children less than 12 months of age who are being treated with methemoglobin-inducing drugs, for safety reasons. Your doctor will not give you the medicine if you have hypersensitivity to lidocaine and / or prilocaine or amide-type local anesthetics or other common ingredients of the cream.
Prilox cream has no or negligible effect on the ability to drive and use machines when used at the recommended doses.
Drug interaction - Drugs that reduce the clearance of lidocaine (eg, cimetidine or beta-blockers); class III antiarrhythmics (such as amiodarone); high doses of Prilox cream may cause additional systemic toxicity; high doses of prilocaine may increase methemoglobin levels, particularly in patients treated with methemoglobin-inducing drugs.
Prilox cream Side effects - Some of the common side effects of the medicine are burning sensation of the skin, Hypersensitivity, Irritation of the cornea, Methemoglobinemia, Purpura, Pruritus, and Petechia at the application site, redness, swelling, and pale skin.
The most commonly observed adverse reactions are site-related (transient local reactions at the application site) and are reported to be common.
It should not be used in eyes, during pregnancy, and if you are having atopic dermatitis.
Due to insufficient data on its absorption, Prilox cream should not be applied to open wounds (except leg ulcers).
Due to the potentially greater absorption on newly shaved skin, it is important to adhere to the recommended dosage, zone and application time.
Patients treated with class III anti-arrhythmic drugs (eg, amiodarone) should be closely monitored and ECG monitoring should be considered as cardiac effects may be observed.
The generic Emla cream contains macrogol-glycerol hydroxy-stearate, which can cause skin reactions.
Prilox cream 2.5% Interesting facts
- It is used as local anesthesia to healthy skin only.
- After a longer period of application, the anesthetic capacity of the cream decreases for the individual.
- Plasma concentrations have not been determined in patients treated with doses> 10 g
- Prilox cream has been used for the treatment of leg ulcers up to 15 times over a period of 1 to 2 months without loss of efficacy or increase in the number or severity of adverse events.
- No dose reduction is required in elderly patients
- In patients with compromised renal function, dosage reduction is not needed.
- Lidocaine and prilocaine have bactericidal and antiviral properties at concentrations greater than 0.5 - 2%.
Generic Emla Prilox real shots

Full Original Product Annotation
For the use only of a Registered Medical Practitioners or a Hospital or a Laboratory.
Prilox cream or Generic Emla
(Lignocaine and Prilocaine Cream I.P.)
DESCRIPTION:
Prilox Cream (Lignocaine and Prilocaine Cream I.P.) is an emulsion in which oil phase is a eutectic mixture of lignocaine and prilocaine in a ratio of 1:1 by weight. This eutectic mixture has a melting point below the room temperature and therefore both local anaesthetics exist as liquid oil rather than as crystals.
Lignocaine is chemically designated as acetamide, 2-(diethylamino)- N-(2,6-dimethylphenyl), has an octonol water partition ratio of 43 at pH 7.4 and has the following structure: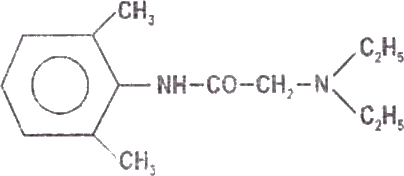
Prilocaine is chemically designated as propanamide, N-(2 methylphenyl)-2-(propylamino), has an octonol water partition ratio of 25 at pH 7.4 and has the following structure: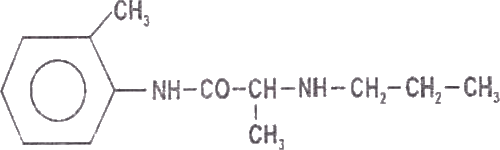
COMPOSITION:
Each gram of Generic Emla (Prilox) contains Lignocaine I.P. 25 mg, Prilocaine I.P. 25 mg.
CLINICAL PHARMACOLOGY:
Mechanism of action: PRILOX Cream (Lignocaine and Prilocaine Cream I.P.), when applied to intact skin under occlusive dressing, provides dermal analgesia by the release of lignocaine and prilocaine from the cream into the epidermal and dermal layers of the skin, leading to accumulation of lignocaine and prilocaine in the vicinity of dermal pain receptors and nerve endings. Both lignocaine and Prilocaine stabilize the neuronal membranes by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby resulting in local anaesthetic action.
INDICATIONS:
PRILOX Cream / Generic Emla (Lignocaine and Prilocaine Cream I.P.) is indicated as a topical anaesthetic for use on normal intact skin only, for local analgesia, for the following procedures:
- Split skin graft harvesting
- Superficial skin surgeries
- Superficial biopsy
- Removal of Molluscum bodies & tattoos
- Electrosurgery of Cutaneous lesions
- Treatment of Condylomata acuminata
- Electrocoagulation
- Premedication for Lignocaine infiltration before skin biopsy.
- Venipuncture
- Intravenous Cannulation
DOSAGE & ADMINISTRATION:
PAEDIATRIC PATIENTS:
The following are the maximum recommended doses and areas of application for PRILOX Cream, based on a child’s age and weight.
|
AGE & BODY WEIGHT REQUIREMENTS |
MAXIMUM TOTAL DOSE OF PRILOX Cream |
MAXIMUM APPLICATION AREA |
|
1-3 months or < 5 kg |
1 g |
10 sq. cm. |
|
4-12 months and > 5 kg |
2g |
20 sq. cm. |
|
1-6 years and > 10 kg |
10 g |
100 sq. cm. |
|
7-12 years and > 20 kg |
20 g |
200 sq. cm. |
Please note: if a patient more than 3 months old does not meet the minimum weight requirements, the maximum total dose of Eutectic mixture of lignocaine and prilocaine should be restricted to that which corresponds to the patient's weight.
Care should be taken to prevent accidental ingestion of Eutectic mixture of lignocaine and prilocaine.
PRILOX Cream should not be used in infants under the age of one month nor in infants under the age of twelve months who are receiving treatment with methemoglobinemia- inducing agents.
ADULT PATIENTS:
A 2 to 3 mm thick layer (approximately 2gm per 10sq cm) of Prilox Cream to be applied on the intact skin under occlusive dressing for 1 to 2 hours.
Dermal analgesia can be expected to increase for up to 3 hrs. under occlusive dressing and persist for 1 to 2 hours after removal of the cream.
CONTRAINDICATIONS:
PRILOX Cream is contraindicated in patients with known history of sensitivity to local anaesthetics of the amide type or any other component of the product.
PRILOX Cream should not be used in those rare patients with congenital or idiopathic methemoglobinemia nor in infants under the age of twelve months who are receiving treatment with methemoglobin inducing agents.
With reference to literature PRILOX Cream is not recommended in any clinical situation, in which penetration or migration beyond the tympanic membrane into the middle ear is possible because of the ototoxic effect observed in animal studies.
PRECAUTIONS:
Generic Emla (Prilox) is not recommended for use on mucous membranes. Safe dosing recommendations for use on mucous membranes cannot be made because it has not been studied adequately.
Repeated doses of PRILOX Cream may increase the blood level of lignocaine and prilocaine. PRILOX Cream should be used with caution in patients who are more sensitive to the systemic effects of prilocaine and lignocaine including acutely ill, debilitated and elderly patients. PRILOX Cream coming in contact with the eyes should be avoided. If eye contact occurs, immediately wash out the eyes with water or saline and protect the eye until sensation returns. PRILOX Cream should be used with caution in patients with history of drug sensitivities, especially if the etiologic agent is uncertain.
Patients with severe hepatic disease, because of their inability to metabolize local anaesthetics, normally are at a greater risk of developing toxic plasma concentration of lignocaine and prilocaine.
Keep out of reach of children.
DRUG INTERACTIONS:
Agents inducing methemoglobinemia, such as:
Analgesics: Acetaminophen, Acetanilide, Phenacetin
Anaesthetics: Benzocaine
Anticonvulsants: Phenobarbital, Phenytoin
Antimalarial Agents: Chloroquine, Pamaquine, Primaquine, Quinine
Sulfonamides / sulfones: Dapsone, Sulfamethoxazole. Trimethoprim Nitrates: Nitrates and nitrites, Nitrofurantoin, Nitroglycerin, Nitroprusside
Other drugs I chemicals: Aniline dyes, Naphthalene, Para-aminosalicylic acid.
PRILOX Cream should be used with caution in patient receiving class I antiarrhythmic drugs (such tocainide and mexiletine), since the toxic effects are additive and potentially synergistic.
WARNING:
Application of PRILOX Cream to larger areas or for longer durations than those recommended could result in sufficient absorption of lignocaine and prilocaine resulting in serious adverse effects.
USE IN PREGNANCY:
Reproductive studies conducted in rats with Lignocaine and Prilocaine have revealed no evidence of impaired fertility or harm to the fetus. However, since there are no adequate or well controlled studies in pregnant women, PRILOX Cream should be used in pregnancy only if clearly needed.
USE IN LABOUR AND DELIVERY:
Neither lignocaine nor prilocaine are contraindicated in labor and delivery. Should PRILOX Cream be used concomitantly with other products containing lignocaine and/or prilocaine, total dose contributed by all the formulation must be considered.
NURSING MOTHERS:
lignocaine and probably prilocaine, are excreted in human milk. Hence, caution should be exercised.
PAEDIATRIC USE:
PRILOX Cream (Generic Emla) should be used with care in patients taking therapy associated with methemoglobinemia. While using Generic Emla in young children, especially infants under the age of three months, care must be taken to limit the area and dose of application, and to prevent ingestion.
In children above one month of age weighing less than 20 kg, the area and duration of application should be limited.
SIDE EFFECTS:
Localised Reaction: During or immediately after treatment with PRILOX Cream, the skin at the site of treatment may develop erythema or edema or may be the locus of abnormal sensation. Rare cases of hyperpigmentation following the use of Generic Emla have been reported.
Allergic Reaction: Allergic and anaphylactic reactions associated with lignocaine and prilocaine can occur. They are characterized by urticaria, angioedema, bronchospasm and shock. If they occur, they should be managed by conventional means.
Systemic (Dose Related) Reactions: Systemic adverse reactions following appropriate use of PRILOX Cream are unlikely due to the small dose absorbed. Systemic adverse effects of lignocaine and or prilocaine are similar to those of other amide type of local anaesthetic agents including CNS excitation and/or depression (light headedness, nervousness, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat or cold or numbness, twitching, tremors, convulsion, unconsciousness, respiratory depression and arrest). Excitatory CNS reactions may be brief or may not occur at all, in which case the first manifestation may be drowsiness merging into unconsciousness. Cardiovascular manifestation may include bradycardia, hypotension and cardiovascular collapse leading to arrest.
OVERDOSAGE:
Toxic levels of lignocaine (>5µg/ml) and / or Prilocaine (>6µg/ml) cause a decrease in cardiac output, total peripheral resistance and mean arterial pressure. These changes may be attributed to the direct depressant effects of these local anaesthetic agents on the cardiovascular system. In the absence of massive topical overdose or oral ingestion, evaluation should include evaluation of other etiologies for the clinical effects or overdosage from other sources of lignocaine, prilocaine or other local anaesthetics.
STORAGE:
Keep the tube tightly closed at all times when not in use. Store below 30°C. Do not freeze.
APPLICATION:
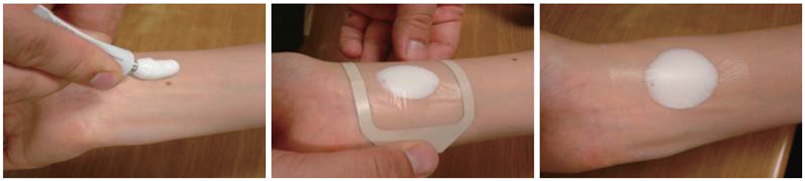
It is very important to follow exactly the instructions given below:
- Squeeze the appropriate quantity (refer to dosage instructions) of the cream into a mound on the site, spread the cream with the help of spatula to form a thick even layer of 2-3 mm. DO NOT RUB THE CREAM IN.
- Carefully cover the layer of PRILOX Cream (Generic Emla) with occlusive dressing and do not compress the cream under the dressing.
- Smooth down the edges of the occlusive dressing to the skin, using a surgical adhesive tape. Leave PRILOX Cream with occlusive dressing for minimum 1-2 hrs. as per the need.
- Before the medical procedure, remove the dressing and then sterilize that area. No sterilization is required before application of the Cream.
PRILOX application
- 1 Shave the skin of the selected site. Clean the area with alcohol.

- 2 Select the exact site and mark this area using indelible thick marker per to delineate the margins of the site.
- 3 Squeeze out Cream from PRILOX tube on the area to be anaesthetized.
- 4 Spread a layer of 2-3rrm thickness of PRILOX with a spatula inside the marked margins.

- 5 Carefully apply the occlusive dressing to completely cover the layer of PRILOX Cream Do not compress.

- 6 Tape down the edges of occlusive dressing with surgical tape & leave it for 1-2 hrs, as per the need.

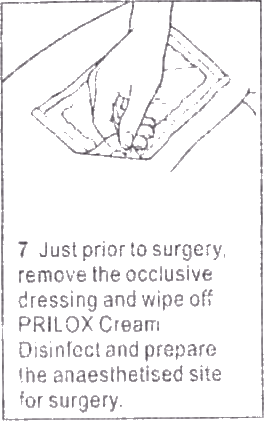
- 7 Just prior to surgery, remove the occlusive dressing and wipe off PRILOX Cream Disinfect and prepare the anaesthetized site for surgery
PRESENTATION:
PRILOX Cream is supplied as 5 gm, 10 gm and 30 gm tube. Each gram of Prilox contains Lignocaine I.P. 25 mg and Prilocaine I.P. 25 mg in Water-Miscible base.
MARKETED ВУ
NEON LABORATORIES LIMITED
28, Mahal Ind. Estate, M. Caves Road,
Andheri (East), Mumbai - 400 093.